What Is Glutathione? A Summary of Glutathione
Glutathione, known as the master antioxidant within cells, is a tripeptide made of glutamine, cysteine, and glycine [1]. Cysteine, the central amino acid in glutathione, contains a carbon atom that is bonded to a sulfur-hydrogen functional group. This arrangement of atoms enables glutathione to perform many functions in the cell.
For example, glutathione can easily attach and detach from other glutathione molecules and other proteins that have this functional group. This characteristic allows the same glutathione molecule to be used repeatedly as an antioxidant, thus enhancing its utility.
Further, this arrangement of atoms explains how glutathione is able to act as a cellular signaling agent. Glutathione signaling helps control reactive oxygen species (ROS), which can damage the body and kill cells.
This atomic arrangement also permits glutathione to bond to other cysteine-containing proteins, which allows it to regulate multiple processes, including metabolic pathways, calcium balance, cellular remodeling, and protein folding. Glutathione can also cause changes to gene expression, DNA and protein synthesis, cell division and growth, and programmed cell death (apoptosis).
Glutathione’s functional group helps it to bind to heavy metals and carcinogens, thus allowing for their removal [2].
In addition, glutathione is an essential building block in the synthesis of several compounds, including leukotrienes and prostaglandins. These molecules are involved in the regulation of the inflammatory response and numerous other balancing functions [3].
The case for boosting glutathione and related enzymes
Glutathione assumes many roles, and its depletion is observed in aging and many chronic diseases [4-6]. To make matters worse, many people have genetic disorders related to the use of glutathione. Often, these individuals possess dysfunctional mutations in one or more enzymes related to glutathione function.
This is prevalent in the glutathione S-transferase (GST) family of genes. In certain populations, 20-50% of individuals possess GST-related enzyme mutations or deletions [7]. Under normal circumstances, these enzymes catalyze glutathione’s detoxifying abilities.
GST helps to manage free radical damage in multiple contexts [8,9]. Increasing glutathione and its supporting enzymes has been shown to reduce oxidative damage and help remove toxic substances from the body.
Research on dietary changes to increase glutathione
There is limited data regarding the effects that different diets have on glutathione activity. A 2018 study found that people who ate a Mediterranean diet had higher plasma glutathione than people who didn’t [10].
Several studies have examined the effects of eating fruits and vegetables on glutathione and its related enzyme levels [11]. Certain vegetables and fruits, including broccoli, garlic, and oranges, may increase the production of GST-related enzymes [12-16].
Substances like sulforaphane, found in broccoli and related vegetables, may increase glutathione and its related enzymes. They may even increase the body’s natural production of antioxidant enzymes [12,17,18].
This is especially relevant to individuals with poorly functioning GST enzymes. Cruciferous vegetables such as broccoli increase GST levels, especially in males.
In another study, young smokers who ate 250 grams of steamed broccoli daily reduced DNA damage by 41%. Additionally, the researchers reported a 23% decrease in DNA strand breaks caused by hydrogen peroxide [19]. Even higher protection was enjoyed by people with a nonfunctional GSTM1 gene.
Drinking fruit and vegetable juices may also be helpful. These juices contain polyphenols that reduce oxidative stress and increase glutathione peroxidase [20-22], an enzyme that works with glutathione to change hydrogen peroxide (a source of oxidative stress) into water.
In a study of weightlifters, pomegranate juice reduced oxidative stress and increased glutathione peroxidase by 6.8% following intense exercise [22]. In another study, participants drank 400 mL of grape juice, organic grape juice, or water daily. Drinking both grape juices led to significantly increased glutathione and glutathione peroxidase compared with water [23,24].
Here are some foods that either directly provide glutathione or stimulate the body to produce glutathione [25,26]:
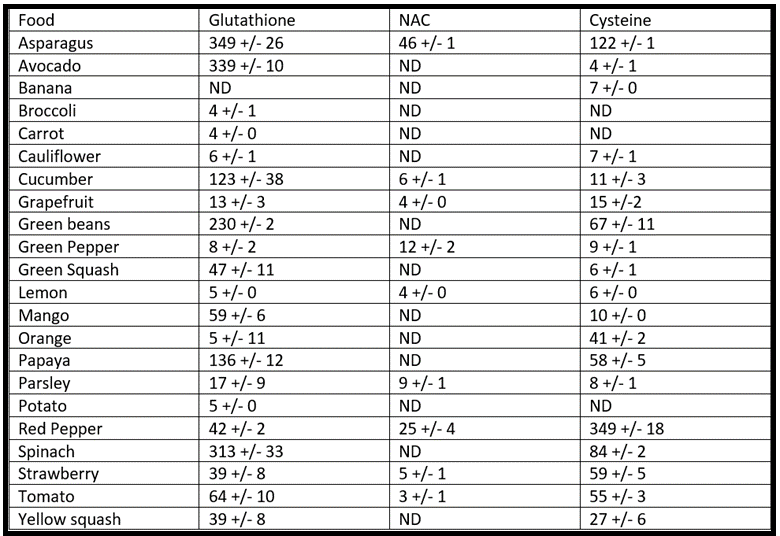
Raw or steamed consumption has been shown to improve the absorption of these nutrients [27]. Fresh is better than frozen, especially with cruciferous vegetables [28].
Additionally, certain herbs may be beneficial. Rosemary [29], curcumin [30], milk thistle [31,32], mustard seed [33], and ginkgo biloba [34] increase glutathione levels in animal studies. Adding mustard seed when cooking cruciferous vegetables improves sulforaphane [31].
Oral glutathione supplementation
Glutathione
The most obvious method for increasing glutathione reserves is direct supplementation. As a tripeptide, glutathione is subject to destruction in the small intestine [35]. Some studies have shown no change in glutathione levels or measurements of oxidative stress despite ongoing glutathione supplementation [36].
Other studies have found that taking glutathione at 250 or 1000 mg/day led to increased glutathione levels [37]. This finding was accompanied by a decrease in oxidative stress indicators.
More recent studies suggest that glutathione is much more easily absorbed when taken in a liposomal or sublingual form. In one study, participants took 500 or 1000 mg per day. After two weeks, plasma concentrations of glutathione were increased by 25% [38]. These approaches appear to protect glutathione from the action of digestive enzymes [39].
N-acetylcysteine (NAC)
The three amino acids that form glutathione do so in two steps. One enzyme catalyzes the bonding of glutamate to cysteine, and then another enzyme catalyzes the bonding of glutamate and cysteine to glycine [40]. Therefore, the body requires both the amino acids and connection-facilitating enzymes to make enough glutathione [37].
Cysteine contains sulfur. This suggests that eating foods high in sulfur and sulfur-containing amino acids could also help glutathione production, as a lack of cysteine is thought to be its main bottleneck [41].
N-acetylcysteine (NAC) is often recommended to enhance glutathione production because it is a source of cysteine [42]. Although NAC could potentially improve glutathione levels and alleviate oxidative stress, this issue is controversial [43-45]. NAC is an antioxidant. This makes it unclear if the effects of NAC on oxidative stress are due to NAC or increased glutathione.
Dietary protein considerations
Impaired protein digestion can endanger healthy glutathione levels. This is often caused by reduced acid production in the stomach or a lack of pancreatic enzymes. People who are showing the signs and symptoms of low albumin and glutathione levels are advised to seek medical help.
Since glutathione is made from amino acids, intake of dietary protein may influence the body’s ability to make glutathione. Reductions in protein consumption [46], even within what are considered safe levels, can alter plasma glutathione synthesis. This, in turn, contributes to a reduction in antioxidant capacity. Whey protein, when needed, can be helpful due to its higher cysteine content [47].
There are potentially other amino acids beyond the glutathione precursors that support glutathione synthesis. Animal studies indicate that serine may increase glutathione production by increasing the availability of cysteine while also reducing epigenetic changes associated with tumor development. Alternatively, serine can be converted to glycine for glutathione synthesis [48,49].
Omega-3 fatty acids
Chronic inflammation can contribute to oxidative stress and deplete glutathione reserves [50]. Omega-3 fatty acids have been shown to reduce inflammation and have an impact on glutathione levels. One study found that women with a GST-related gene deletion benefited from eating large amounts of fish high in omega-3. Those who did had a 64% reduction in the risk of breast cancer [51].
Vitamins B, C, and E
Riboflavin is a necessary coenzyme to activate glutathione reductase. This enzyme converts oxidized glutathione into its reduced form. This is needed for glutathione to function as an antioxidant and explains how riboflavin deficiency can depress its function [52].
Pantothenic acid (vitamin B5) has been shown to increase glutathione synthesis by improving cellular energetics [53].
Adequate B12 is also important, as deficiencies of B12 have been shown to reduce glutathione [54]. 500 to 1000 mg of vitamin C per day has been shown to increase lymphocyte and red blood cell glutathione by 18% [55,56]. Vitamin E supplementation in diabetics has been shown to increase glutathione by up to 9% [57].
Alpha-lipoic acid
Alpha-lipoic acid is a scavenger of free radicals and also aids in the regeneration of glutathione. HIV-infected adults in one study were given 300 mg of alpha-lipoic acid three times daily for six months, which resulted in significant elevations in total glutathione [58].
Selenium
Selenium is an antioxidant that works alongside glutathione peroxidase, which helps glutathione convert hydrogen peroxide to water. In a mouse study, selenium supplementation increased the expression and activity of certain enzymes related to glutathione [59].
Side effects of glutathione
Few side effects are associated with dietary changes aimed at elevating glutathione reserves. One study looked at thyroid cancer in women from New Caledonia and found a relationship between thyroid cancer and high cruciferous vegetable intake in the presence of low iodine levels [60]. Otherwise, mild abdominal discomfort and flatulence associated with raffinose in cruciferous vegetables may be anticipated [61]. There are no side effects or mentions of glutathione toxicity in the academic literature.
Selenium toxicity can occur with excessive intake [62]. If you do experience any adverse effects, you should cease taking it immediately and consult your doctor.
Summary of recommendations from studies on boosting glutathione
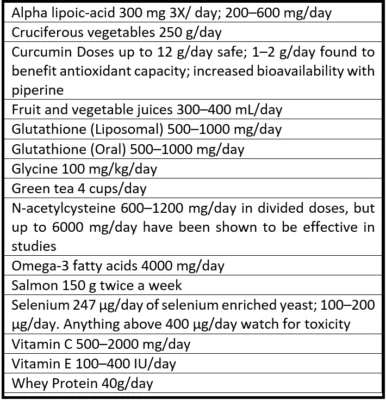 The table to the left was developed by Minich et al. and provides a list of recommendations compiled from academic journals. These findings examine the effects of food and supplementation of boosting glutathione [26].
The table to the left was developed by Minich et al. and provides a list of recommendations compiled from academic journals. These findings examine the effects of food and supplementation of boosting glutathione [26].
Disclaimer
This article is only a very brief summary. It is not intended as an exhaustive guide and is based on the interpretation of research data, which is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you. We do not endorse supplement use or any product or supplement vendor, and all discussion here is for scientific interest.
Literature
[1] M. H. Hoffmann and H. R. Griffiths, “The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models,” Free Radic. Biol. Med., vol. 125, no. March, pp. 62–71, 2018
[2] T. Okuyama and H. Maskill, Organic Chemistry A Mechanistic Approach. Oxford, United Kingdom: Oxford University Press, 2014.
[3] G. Wu, Y. Z. Fang, S. Yang, J. R. Lupton, and N. D. Turner, “Glutathione Metabolism and Its Implications for Health,” J. Nutr., vol. 134, no. 3, pp. 489–492, 2004
[4] M. Erden-Inal, E. Sunal, and G. Kanbak, “Age-related changes in the glutathione redox system,” Cell Biochem. Funct., vol. 20, no. 1, pp. 61, 2002
[5] R. V. Sekhar et al., “Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation,” Am. J. Clin. Nutr., vol. 94, no. 3, pp. 847–853, 2011
[6] P. Kumar et al., “Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Re,” Clin. Transl. Med., vol. 11, no. 3, 2021
[7] A. L. Hollman, P. B. Tchounwou, and H. C. Huang, “The association between gene-environment interactions and diseases involving the human GST superfamily with SNP variants,” Int. J. Environ. Res. Public Health, vol. 13, no. 4,pp.1-14, 2016
[8] C. Ott, K. Jacobs, E. Haucke, A. Navarrete, T. Grune, and A. Simm, “Redox Biology Role of advanced glycation end products in cellular signaling,” Redox Biol., vol. 2, pp. 411–429, 2014
[9] I. Liguori et al., “Oxidative Stress, Aging and Diseases,” Oxidative Stress Dis., pp. 757–772, 2012
[10] E. L. Bettermann et al., “Higher Mediterranean Diet Quality Scores and Lower Body Mass Index Are Associated with a Less-Oxidized Plasma Glutathione and Cysteine Redox Status in Adults,” J. Nutr., vol. 148, no. 2, pp. 245–253, 2018
[11] P. A. Wark et al., “Habitual consumption of fruits and vegetables: Associations with human rectal glutathione S-transferase,” Carcinogenesis, vol. 25, no. 11, pp. 2135–2142, 2004
[12] M. Clapper, C. Szarka, and P. Engstrom, “Preclinical and clinical evaluation of broccoli supplements as inducers of glutathione S-transferase activity,” Clin. Cancer Res., vol. 3, no. 1, pp. 25–30, 1997
[13] W. A. Nijhoff et al., “Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans,” Carcinogenesis, vol. 16, no. 9, pp. 2125–2128, 1995
[14] J. Lampe, C. Chen, J. Prunty, and J. Potter, “Modulation of human glutathione S-transferases by botanically defined vegetable diets,” cancer epidemiol biomarkers prev, vol. 9, no. 787–793, 2000
[15] J. J. P. Bogaards, H. Verhagen, M. I. Willems, G. Van Poppel, and P. J. va. Bladeren, “Consumption of brussels sprouts results in elevated a-class glutathione s-transferase levels in human blood plasma,” Carcinogenesis, vol. 15, no. 5, pp. 1073–1075, 1994
[16] S. Hasegawa and M. Miyake, “Biochemistry and biological functions of citrus limonoids,” Food Rev. Int., vol. 12, no. 4, pp. 413–435, 1996
[17] A. F. Abdull Razis, N. Konsue, and C. Ioannides, “Isothiocyanates and Xenobiotic Detoxification,” Mol. Nutr. Food Res., vol. 62, no. 18, pp. 1–35, 2018
[18] D. O. Saleh, D. F. Mansour, I. M. Hashad, and R. M. Bakeer, “Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway.,” Eur. J. Pharmacol., vol. 855, no. 4 pp. 40–49, 2019
[19] P. Riso et al., “DNA damage and repair activity after broccoli intake in young healthy smokers,” Mutagenesis, vol. 25, no. 6, pp. 595–602, 2010
[20] Z. Pourahmadi, S. Mahboob, A. Saedisomeolia, and M. T. Reykandeh, “The Effect of Tomato Juice Consumption on Antioxidant Status in Overweight and Obese Females,” Women Heal., vol. 55, no. 7, pp. 795–804, 2015
[21] O. D. Rangel-Huerta et al., “Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults,” J. Nutr., vol. 145, no. 8, pp. 1808–1816, 2015
[22] A. Ammar et al., “Effects of Pomegranate Juice Supplementation on Oxidative Stress Biomarkers Following Weightlifting Exercise,” Nutrients, vol. 9, no. 8, p. 819, 2017
[23] I. M. Toaldo, F. A. Cruz, E. L. da Silva, and M. T. Bordignon-Luiz, “Acute consumption of organic and conventional tropical grape juices (Vitis labrusca L.) increases antioxidants in plasma and erythrocytes, but not glucose and uric acid levels, in healthy individuals,” Nutr. Res., vol. 36, no. 8, pp. 808–817 2016
[24] S. Galland and I. Stamenkovic, “Mesenchymal stromal cells in cancer?: a review of their immunomodulatory functions and dual effects on tumor progression S Galland and I Stamenkovic,” Journal of Pathology.,pp. 555–572, 2019
[25] O. Demirkol, C. Adams, and N. Ercal, “Biologically important thiols in various vegetables and fruits,” J. Agric. Food Chem., vol. 52, no. 26, pp. 8151–8154, 2004
[26] D. M. Minich and B. I. Brown, “A review of dietary (Phyto)nutrients for glutathione support,” Nutrients, vol. 11, no. 9, pp. 1–20, 2019
[27] I. Sarvan, E. Kramer, H. Bouwmeester, M. Dekker, and R. Verkerk, “Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli,” Food Chem., vol. 214, pp. 580–586, 2017
[28] E. B. Dosz and E. H. Jeffery, “Modifying the Processing and Handling of Frozen Broccoli for Increased Sulforaphane Formation,” J. Food Sci., vol. 78, no. 9, pp. H1459–H1463, 2013
[29] A. Raškovic, I. Milanovic, N. Pavlovic, T. Cebovic, S. Vukmirovic, and M. Mikov, “Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential,” BMC Complement. Altern. Med., vol. 14, p. 225, 2014
[30] S. Abrahams, W. L. Haylett, G. Johnson, J. A. Carr, and S. Bardien, “Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review,” Neuroscience, vol. 406, pp. 1–21, 2019
[31] C. Soto, J. Pérez, V. García, E. Uría, M. Vadillo, and L. Raya, “Effect of silymarin on kidneys of rats suffering from alloxan-induced diabetes mellitus,” Phytomedicine, vol. 17, no. 14, pp. 1090–1094, 2010
[32] F. Alidoost et al., “Effects of silymarin on the proliferation and glutathione levels of peripheral blood mononuclear cells from ß-thalassemia major patients,” Int. Immunopharmacol., vol. 6, no. 8, pp. 1305–1310, 2006
[33] O. Okunade, K. Niranjan, S. K. Ghawi, G. Kuhnle, and L. Methven, “Supplementation of the Diet by Exogenous Myrosinase via Mustard Seeds to Increase the Bioavailability of Sulforaphane in Healthy Human Subjects after the Consumption of Cooked Broccoli,” Mol. Nutr. Food Res., vol. 62, no. 18, p. 1700980, 2018
[34] K. Sasaki et al., “Effects of extract of Ginkgo biloba leaves and its constituents on carcinogen-metabolizing enzyme activities and glutathione levels in mouse liver,” Life Sci., vol. 70, no. 14, pp. 1657–1667, 2002
[35] M. K. Hunjan and D. F. Evered, “Absorption of glutathione from the gastro-intestinal tract,” Biochim. Biophys. Acta – Biomembr., vol. 815, no. 2, pp. 184–188, 1985
[36] J. Allen and R. D. Bradley, “Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers,” J. Altern. Complement. Med., vol. 17, no. 9, pp. 827–833, 2011
[37] J. P. Richie et al., “Randomized controlled trial of oral glutathione supplementation on body stores of glutathione,” Eur. J. Nutr., vol. 54, no. 2, pp. 251–263, 2015
[38] S. C. Lu, “Glutathione synthesis,” Biochim. Biophys. Acta – Gen. Subj., vol. 1830, no. 5, pp. 3143–3153, 2013
[39] R. Sinha et al., “Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function,” Eur. J. Clin. Nutr., vol. 72, no. 1, pp. 105–111, 2018
[40] M. F. McCarty, J. H. O’Keefe, and J. J. DiNicolantonio, “Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection,” Ochsner J., vol. 18, no. 1, pp. 81–87, 2018
[41] S. Parcell, “Sulfur in Human Nutrition and Applications in Medicine,” Altern. Med. Rev., vol. 7, no. 1, pp. 22–35, 2002.
[42] K. R. Atkuri, J. J. Mantovani, L. A. Herzenberg, and L. A. Herzenberg, “N-Acetylcysteine–a safe antidote for cysteine/glutathione deficiency,” Curr. Opin. Pharmacol., vol. 7, no. 4, pp. 355–359, 2007
[43] G. F. Rushworth and I. L. Megson, “Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits,” Pharmacol. Ther., vol. 141, no. 2, pp. 150–159, 2014
[44] B. Panizzutti et al., “Mediator effects of parameters of inflammation and neurogenesis from a N-acetyl cysteine clinical-trial for bipolar depression,” Acta Neuropsychiatr., vol. 30, no. 6, pp. 334–341, 2018
[45] V. Paschalis, A. A. Theodorou, N. V Margaritelis, A. Kyparos, and M. G. Nikolaidis, “N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione,” Free Radic. Biol. Med., vol. 115, pp. 288–297, 2018
[46] A. A. Jackson, N. R. Gibson, Y. Lu, and F. Jahoor, “Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein,” Am. J. Clin. Nutr., vol. 80, no. 1, pp. 101–107, 2004
[47] P. Tosukhowong et al., “Biochemical and clinical effects of Whey protein supplementation in Parkinson’s disease: A pilot study,” J. Neurol. Sci., vol. 367, pp. 162–170, 2016
[48] X. Zhou et al., “Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes,” Biochim. Biophys. Acta – Mol. Basis Dis., vol. 1864, no. 2, pp. 488–498, 2018
[49] X. Zhou, L. He, C. Wu, Y. Zhang, X. Wu, and Y. Yin, “Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice,” Mol. Nutr. Food Res., vol. 61, no. 11, p. 1700262, 2017
[50] I. Rahman, “Inflammation and the Regulation of Glutathione Level in Lung Epithelial Cells,” Antioxid. Redox Signal., vol. 1, no. 4, pp. 425–447, 1999
[51] M. Gago-Dominguez et al., “Marine n-3 fatty acid intake, glutathione S -transferase polymorphisms and breast cancer risk in post-menopausal Chinese women in Singapore ,” Carcinogenesis, vol. 25, no. 11, pp. 2143–2147, 2004
[52] M. Ashoori and A. Saedisomeolia, “Riboflavin (vitamin B2) and oxidative stress: a review,” Br. J. Nutr., vol. 111, no. 11, pp. 1985–1991, 2014
[53] V. S. Slyshenkov, D. Dymkowska, and L. Wojtczak, “Pantothenic acid and pantothenol increase biosynthesis of glutathione by boosting cell energetics,” FEBS Lett., vol. 569, no. 1–3, pp. 169–172, 2004
[54] E. Van de Lagemaat, M. de Groot, and E. Van den Heuvel, “Vitamin B(12) in Relation to Oxidative Stress: A Systematic Review,” Nutrients, vol. 11, no. 2, p. 482, 2019
[55] K. J. Lenton, A. T. Sané, H. Therriault, A. M. Cantin, H. Payette, and J. R. Wagner, “Vitamin C augments lymphocyte glutathione in subjects with ascorbate deficiency,” Am. J. Clin. Nutr., vol. 77, no. 1, pp. 189–195, 2003
[56] C. S. Johnston, C. G. Meyer, and J. C. Srilakshmi, “Vitamin C elevates red blood cell glutathione in healthy adults,” Am. J. Clin. Nutr., vol. 58, no. 1, pp. 103–105, 1993, doi: 10.1093/ajcn/58.1.103.
[57] A. Sharma, S. Kharb, S. N. Chugh, R., and G. P. Singh, “Evaluation of oxidative stress before and after control of glycemia and after vitamin E supplementation in diabetic patients,” Metabolism., vol. 49, no. 2, pp. 160–162, 2000
[58] R. J. Jariwalla et al., “Restoration of Blood Total Glutathione Status and Lymphocyte Function Following a-Lipoic Acid Supplementation in Patients with HIV Infection,” J. Altern. Complement. Med., vol. 14, no. 2, pp. 139–146, 2008
[59] E. Song et al., “Selenium supplementation shows protective effects against patulin-induced brain damage in mice via increases in GSH-related enzyme activity and expression,” Life Sci., vol. 109, no. 1, pp. 37–43, 2014
[60] T. Truong, D. Baron-Dubourdieu, Y. Rougier, and P. Guénel, “Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case–control study in New Caledonia,” Cancer Causes Control, vol. 21, no. 8, pp. 1183–1192, 2010
[61] R. H. Bimo Setiarto, N. Widhyastuti, and D. R. Kurnia, “Optimal concentration of prebiotic raffinose to increase viability of Lactobacillus acidophilus, Lactobacillus bulgaricus, Streptococcus thermophilus.,” Carpathian J. Food Sci. Technol., vol. 13, no. 3, pp. 147–157, 2021
[62] I. Galan-Chilet et al., “Plasma selenium levels and oxidative stress biomarkers: A gene-environment interaction population-based study,” Free Radic. Biol. Med., vol. 74, pp. 229–236, 20

