Higher Abdominal Fat Associated with Cognitive Decline
- There are differences between males and females in its impact.

A group of Japanese researchers has published a paper reporting an association between higher abdominal fat levels and cognitive decline [1].
Abdominal fat and brain connection
Aging is often linked to many comorbidities, one of which is dementia, and developing drugs to treat this condition is challenging. An alternative, more immediately feasible approach is to identify modifiable factors that can lower the likelihood of this disease. One such factor is obesity, which has been previously identified [2]. However, there is a twist: the relationship between weight and dementia is not so straightforward. Previous studies have found that while midlife obesity is a dementia risk factor, higher BMI later in life is associated with less dementia [3].
It may, therefore, be possible that BMI is not the best indicator of nutritional status in later life, as the elderly often experience changes in body composition, specifically an increase in fat mass and a decrease in fat-free mass, which does not result in significant change to BMI [4].
To reflect those changes, recent studies have associated different health metrics, such as waist circumference and waist-hip ratio, with dementia [5, 6], suggesting an adverse role of abdominal fat on the brain. However, the roles of specific measurements and the impact of types of fat on cognitive decline is still a subject of debate.
Men’s and women’s visceral fat impact differs
The researchers in the recent study used data from the National Institute for Longevity Sciences’ Longitudinal Study of Aging. This study included community-based older individuals living in Japan. 873 participants were followed up every 2 years for a median follow-up period of 9.67 years. The participants were 60 years and older and didn’t have cognitive impairment at the start of the study.
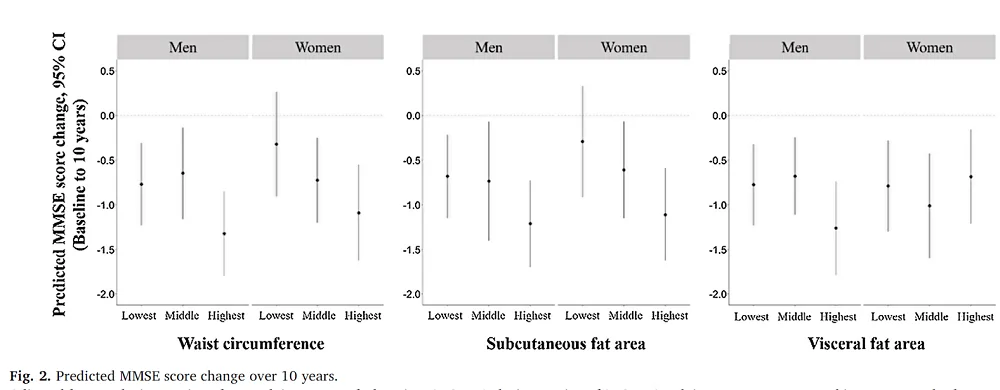
Each participant’s waist circumference, subcutaneous fat area, and visceral fat area were measured, and the Mini-Mental State Examination (MMSE) was conducted to assess their cognitive abilities. Each of these metrics was divided into three groups: lowest, middle, and highest.
With this data in hand, the researchers performed modeling that indicated that for men, the biggest decline in the MMSE is among men with the highest levels of waist circumference, subcutaneous fat area, and visceral fat area when compared to the groups with the lowest levels. In the case of women, the group with the highest waist circumference and subcutaneous fat area also had the biggest declines in MMSE compared to the lowest waist circumference and subcutaneous fat area groups, just like men. However, that was not the case for the visceral fat area groups.
The hormone hypothesis
In their discussion, the authors try to interpret their results and provide possible explanations based on current knowledge. They propose that the association between visceral and subcutaneous fat and cognitive decline might be due to the role that adipose tissue plays as an endocrine organ that secretes adipokines. They believe that “excessive abdominal obesity may increase the secretion of inflammatory cytokines, which may contribute to cognitive decline.” For example, higher levels of one adipokine, the inflammatory cytokine IL-6, were previously reported to be associated with increased dementia risk [7].
Adipose tissue also secretes adipokines, such as leptin and adiponectin, that have neuroprotective and anti-inflammatory functions. Excessive abdominal fat levels are linked to the reduced production of adiponectin. On the other hand, leptin production is increased in such a condition, but its permeability through blood–the blood-brain barrier is decreased [8,9]. Therefore, excessive abdominal fat can lead to weakening of leptin and adiponectin’s protective effects.
The authors also tried to understand the interesting observation that visceral fat area was associated with cognitive decline in men but not women. The authors speculate that estrogen, a female sex hormone that has been demonstrated to have a protective effect against cognitive decline, plays a role in this process [10]. Adipose tissue plays a role in estrogen metabolism [11], and “peripheral adipose tissue may be a source of estrogen in postmenopausal women” [12]. All these studies taken together suggest that visceral fat accumulation in older women increases estrogen levels, which can have a protective effect against cognitive decline. However, his hypothesis, like the previous ones, requires direct testing.
Higher WC, SFA, and VFA in men and higher WC and SFA in women were associated with greater cognitive decline over the subsequent 10 years. However, no association was observed between visceral fat accumulation and cognitive decline in women. These findings suggest that the accumulation of abdominal adiposity is a risk factor for cognitive decline among older adults. Furthermore, the abdominal adiposity involved in cognitive decline may differ between men and women, and further studies are required to understand the mechanisms of this difference between the sexes.
Literature
[1] Uchida, K., Sugimoto, T., Tange, C., Nishita, Y., Shimokata, H., Saji, N., Kuroda, Y., Matsumoto, N., Kishino, Y., Ono, R., Akisue, T., Otsuka, R., & Sakurai, T. (2024). Association between abdominal adiposity and cognitive decline in older adults: a 10-year community-based study. The journal of nutrition, health & aging, 28(3), 100175. Advance online publication.
[2] Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., Brayne, C., Burns, A., Cohen-Mansfield, J., Cooper, C., Costafreda, S. G., Dias, A., Fox, N., Gitlin, L. N., Howard, R., Kales, H. C., Kivimäki, M., Larson, E. B., Ogunniyi, A., Orgeta, V., … Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England), 396(10248), 413–446.
[3] Pedditzi, E., Peters, R., & Beckett, N. (2016). The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age and ageing, 45(1), 14–21.
[4] Jackson, A. S., Janssen, I., Sui, X., Church, T. S., & Blair, S. N. (2012). Longitudinal changes in body composition associated with healthy ageing: men, aged 20-96 years. The British journal of nutrition, 107(7), 1085–1091.
[5] Tang, X., Zhao, W., Lu, M., Zhang, X., Zhang, P., Xin, Z., Sun, R., Tian, W., Cardoso, M. A., Yang, J., Simó, R., Zhou, J. B., & Stehouwer, C. D. A. (2021). Relationship between Central Obesity and the incidence of Cognitive Impairment and Dementia from Cohort Studies Involving 5,060,687 Participants. Neuroscience and biobehavioral reviews, 130, 301–313.
[6] Arnoldussen, I. A. C., Gustafson, D. R., Leijsen, E. M. C., de Leeuw, F. E., & Kiliaan, A. J. (2019). Adiposity is related to cerebrovascular and brain volumetry outcomes in the RUN DMC study. Neurology, 93(9), e864–e878.
[7] Darweesh, S. K. L., Wolters, F. J., Ikram, M. A., de Wolf, F., Bos, D., & Hofman, A. (2018). Inflammatory markers and the risk of dementia and Alzheimer’s disease: A meta-analysis. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 14(11), 1450–1459.
[8] Forny-Germano, L., De Felice, F. G., & Vieira, M. N. D. N. (2019). The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Frontiers in neuroscience, 12, 1027.
[9] Kim, J. Y., Barua, S., Jeong, Y. J., & Lee, J. E. (2020). Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer’s Disease. International journal of molecular sciences, 21(17), 6419.
[10] Engler-Chiurazzi, E. B., Brown, C. M., Povroznik, J. M., & Simpkins, J. W. (2017). Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Progress in neurobiology, 157, 188–211.
[11] Mahabir, S., Baer, D. J., Johnson, L. L., Hartman, T. J., Dorgan, J. F., Campbell, W. S., Clevidence, B. A., & Taylor, P. R. (2006). Usefulness of body mass index as a sufficient adiposity measurement for sex hormone concentration associations in postmenopausal women. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 15(12), 2502–2507.
[12] Gruzdeva, O., Borodkina, D., Uchasova, E., Dyleva, Y., & Barbarash, O. (2019). Leptin resistance: underlying mechanisms and diagnosis. Diabetes, metabolic syndrome and obesity : targets and therapy, 12, 191–198.







