Hard Science and Long-Shot Ideas Meet in Vitalia
- This conference held in a longevity pop-up city had something for everyone.

Vitalia, the longevity pop-up city that came into being earlier this year on the island of Roatan off the coast of Honduras, was a first-of-its-kind event that we will more extensively cover later. Today, we are happy to present a roundup of the talks from the longevity biology conference held in Vitalia on the 23rd and 24th of last month. This conference was organized by Lifespan.io’s longtime ally, VitaDAO.
As usual, we can only bring you a fraction of these talks. The process of selection was painful and somewhat arbitrary, and we customarily apologize to those incredibly worthy participants who didn’t make the cut. The entirety of the talks can be accessed here.
Biology, flip-flops, and coconuts
Last year’s pop-up city, Zuzalu, which we also covered extensively, was a groundbreaking experiment in co-living that might one day pave the way for a full-blown network state. Given its resounding success, it’s no wonder that some of the elements test-driven in Zuzalu, such as on-site science conferences, are becoming a staple in similar new projects.
Conferences attract top talent with the promise of a previously unheard-of mix of professional networking and leisure activities in an exotic location. While a normal conference only lasts a couple of days, here, a scientist or a biotech investor can simultaneously enjoy the company of like-minded people and the pleasures of scuba diving for much longer. The big names coming to share their knowledge and get some tan attract, in turn, the rest of the crowd which forms the financial backbone of the whole undertaking.
The closing longevity conference in Vitalia (there were two of them), just like its Montenegrin predecessor, was cheerfully informal. For instance, there was no dress code: some speakers trotted the stage in briefs and sandals, while others opted for a less casual look, all the way up to a full suit.
The stage was decorated with palm leaves and potted plants, and the first rows of seats consisted of red and black bean bags, so participants would be listening to scientific talks in full recline mode while sipping from freshly harvested coconuts.
The daring atmosphere of such conferences also allows experimenting with content, bringing in topics that are mostly excluded from the bigger and more buttoned-up events. This can range from areas that fall outside the geroscience envelope, such as longevity advocacy or space medicine, to topics considered by some too extreme and outlandish, like cryopreservation or full-body replacement.
One of the conference’s organizers, Max Unfried of VitaDAO and the National University of Singapore, expanded on this in his opening address. The goal of the Vitalia conference, he said, was to speed up the discovery of longevity interventions, which is also Vitalia’s ultimate aim. To achieve this, we need to not shy away from new research pathways, however long the odds may seem. We also need to distribute our efforts across a wide range of avenues, including improvements in regulation and garnering popular support. Aging must be attacked from multiple angles simultaneously. Meanwhile, we must stay healthy, keep calm, and carry on.
Max Unfried opens the conference
Live long and Prospera
In his talk, Niklas Anzinger, co-founder of Vitalia, briefly outlined its main tenets. Vitalia, according to him, will not vanish from the face of the Earth, but is going to become a lasting “city that builds longevity companies”. The unique regulatory climate of Prospera, the free economic zone that Vitalia is nested within, can speed up research and help biotech companies survive by cutting trial costs. Niklas characterized Prospera legal system, which includes novel concepts like crypto-native, tokenized property rights, as “the most innovative in the world”, a paragon of regulatory flexibility and medical freedom. According to Niklas, this makes it possible to bring drugs to the market by spending as little as four months and one million dollars, as opposed to 10+ years and upwards of two billion dollars, which is the case today.
Prospera’s laws allow companies to start selling their therapies to clients after completion of Phase I trials, which establish the safety profile, rather than Phase II trials which test the efficacy. People who believe in the underlying science are welcome to come to Honduras and spend your hard-earned money on a yet-unproven therapy. This allows companies to escape “the valley of death” – the long and costly period of bringing their novel therapy to the clinic. Off-label drugs, Niklas reminded the audience, have only safety data and yet are allowed on the market, so why discriminate against new therapies?
Niklas reported that Vitalia has already surpassed its initial goals by incubating 38 companies, instead of 15 as was planned, and enlisting 25-30 permanent residents. In the next two years, Vitalia is planned to grow to 500-1000 residents, and at least one additional permanent chapter will open elsewhere in the world. By 2034, the plan is to have 3-5 districts, 1000+ new products, 100.000+ residents and more than 50 billion dollars in equity and land, putting Vitalia firmly on the course of becoming a true longevity network state.
Do we need another “ism”? Yes!
Adam Gries is a co-founder of Vitalism (not to be confused with Vitalia), another movement that aims to unite longevity enthusiasts, create a permanent presence somewhere on the globe, and facilitate longevity research. Last year, Vitalism held its first conference in Rhode Island, one of the places Vitalists had marked as a possible bridgehead due to its high development level and small population size that make influencing policies via elections easier.
Vitalists think big, pointing at past and present situations in which humanity proved able to pull off large-scale projects such as the Apollo program or deciphering the human genome. Humanity’s failure to assign the same degree of importance and urgency to the fight against aging is paradoxical and must be rectified.
Adam recited the Vitalism Declaration, which starts with the words “Life and health are good” – apparently, something our movement has to defend. The declaration calls for work towards reaching “unlimited healthy human lifespan”, an idea that was considered fringe in the longevity space just a while ago but is currently hailed by many individuals and organizations.
The main ideological difference between Vitalism and Vitalia, according to Adam, is that Vitalists assign a lot of importance to recruiting today’s nation-states to the fight by changing public opinion: “Vitalia believes that freedom is the primary channel to achieve breakthroughs”, he said. “I’m more agnostic. It’s great to apply massive resources everywhere we can, trying to change things inside nation-states.”
Better packaging matters
As the conference pivoted towards hard science, Vera Gorbunova, University of Rochester professor and one of the world’s most prominent geroscientists, took the stage.
In Vera’s view, “the most causal hallmarks (of aging) are those that deal with genome and epigenome”, so, reversing genomic and epigenomic instability can potentially deal a serious blow to aging. SIRT6, she said, is essentially “a packaging protein” that packs heterochromatin tightly to avoid unwanted transcription. As SIRT6 gets dysfunctional with age, transposable elements (transposons), such as LINE1, the most abundant of them all, start being transcribed, triggering immune reaction and inflammation. In a healthy organism, transposons seem to be specifically silenced. Vera teased unpublished results suggesting extended healthspan in mice with LINE1 knocked down.

Vera Gorbunova cheerfully presents the yet unpublished results
SIRT6 is more active in many long-lived species, which seems to be an evolutionarily conserved strategy. Upregulating or otherwise improving SIRT6 can lead to epigenetic rejuvenation as “a more conservative strategy versus Yamanaka (factors)”.
This can be achieved by gene therapy (giving people extra copies of SIRT6 or using its more effective versions found in long-lived species), but small molecules are a viable and simpler option. Vera’s group discovered that fucoidan, a molecule abundant in edible seaweed, boosts SIRT6 activity, increasing healthspan and lifespan in mice. For a deeper dive, read our last year’s interview with Vera.
Naked mole… mice?
Usually, Vera Gorbunova and her lifetime partner and collaborator Andrei Seluanov, also professor at Rochester, divide their conference appearances between them, but in Vitalia, the well-rested couple took the stage one after the other.
Andrei also studies long-lived species. One of these projects involves hyaluronic acid, a substance mostly known to us from the cosmetic industry, specifically, the more active version of hyaluronan found in naked mole rats (NMR). Supposedly, the tweaked hyaluronan provides NMRs with resistance to cancer and many other age-related diseases.
Andrei described creating transgenic mice that express the NMR version of hyaluronan, a feat that took ten years to achieve. Those mice live longer and exhibit less frailty. Their transcriptome is different from that of wild type mice in a way that resembles long-lived species (“they are NMRs on a transcriptional level”, Andrei said). The animals also have reduced autoimmune response and inflammation.
Another animal Andrei studies sits on the opposite side of the body size scale – the bowhead whale, supposedly the longest-lived mammal with a lifespan in excess of two centuries. Living that long and weighing 60 tons, how do those huge animals with many more cells than in our bodies evade cancer?
Unlike elephants, bowhead whales do not dramatically overexpress p53, the protein that kills precancerous cells, and they require even fewer mutations to achieve cancer than humans – meaning, their anti-cancer protections must be of a different nature.
It turns out that bowhead whales have superior DNA repair mechanisms, meaning that their cells do not accumulate enough oncogenic mutations to become cancerous in the first place. One of the proteins that regulate this mechanism is called CIRBP, and the first two letters in its name stand for “cold-induced”. Andrei’s talk complemented Vera’s, and both painted a convincing picture of how long-lived species differ from us and what we can do to become more like them.

Andrei Seluanov – on rats and whales
The importance of reproductive aging
Marina Marinova of AthenaDAO, a decentralized science organization devoted to women’s health research, gave an important overview of the phenomenon of ovarian aging. Humans are among the handful of species that experience menopause, the shutdown of the female reproductive system that comes decades before other systems and organs start giving up.
While it is a simplification, in general, women tend to age slower than men before menopause and faster after menopause (centenarians and supercentenarians are still overwhelmingly women). Interestingly, while female life expectancy has been on the rise, the age of menopause has hardly budged, and today, women spend about 40% of their lifespans after menopause, i.e., in poorer health, prone to various age-related diseases.
Later menopause is known to correlate with longer lifespan, which makes studying and countering female reproductive aging so important. Some of the current approaches include such “crowd favorites” in the longevity field as NAD+ and rapamycin. Marina also mentioned more controversial ideas such as the hunt for the newly discovered germline stem cells, or “oogonial stem cells”, which Marina compared to the Loch Ness monster since there is still some doubt about their very existence.
AthenaDAO crowdfunds research into ovarian aging with IP-NFTs, including projects such as Mario Cordero’s study into inflammation, supposedly a major cause of ovarian aging. Ovarian tissue replacement is being studied as well. As Marina reported, transplanting young mouse ovaries into old mice increases their lifespan.
Nicolina Lauc of GlycanAge gave an interesting glycan-centered perspective on reproductive aging. Glycans are complex carbohydrates or polysaccharides consisting of chains of sugar molecules. They play crucial roles in various biological processes and are found throughout all living organisms. Glycans modify proteins through glycosylation, and the glycan landscape, unique for every human, can be indicative of various diseases, including diseases of aging, sometimes years before the diagnosis. Glycans also regulate inflammation.
Apparently, during menopause, the glycan profile changes, which indicates post-menopausal health problems. GlycanAge scientists were also able to detect a beneficial effect of hormone replacement therapy (HRT). Results of a yet unpublished study indicate that HRT significantly changes pro-inflammatory glycans in a way that decreases biological age. This suggests that glycans can be used to track menopause and optimize interventions.
This and other findings have led to the idea that estrogen might be a longevity drug. Interestingly, the interplay between testosterone and estrogen seems to affect aging in men as well. Of note, results from the ITP (Intervention Testing Program) showed considerable lifespan extension in male mice who received 17-alpha-estradiol, also known as a “non-feminizing estrogen”.
Should we expect true mortality postponement?
The two talks dedicated to “the oldest old” – centenarians and supercentenarians – were among the most exciting at the conference. Biological and statistical analysis of this small population can yield important insights into human aging and the limits of human lifespan.
Robert Young of the Gerontology Research Group (GRG) spent many years trying to understand how long people actually live. According to him, two largest datasets on the oldest old – the GRG dataset and IDL (International Database on Longevity) “firmly conclude that there is a statistically significant maximum human lifespan, theoretically estimated to be around age 125, with environmental effects included”. However, it is “an obstacle to be overcome, not a dogmatic barrier”. As tentative evidence to that, “a look at maximum observed human mortality is showing a gradual upward trend”:
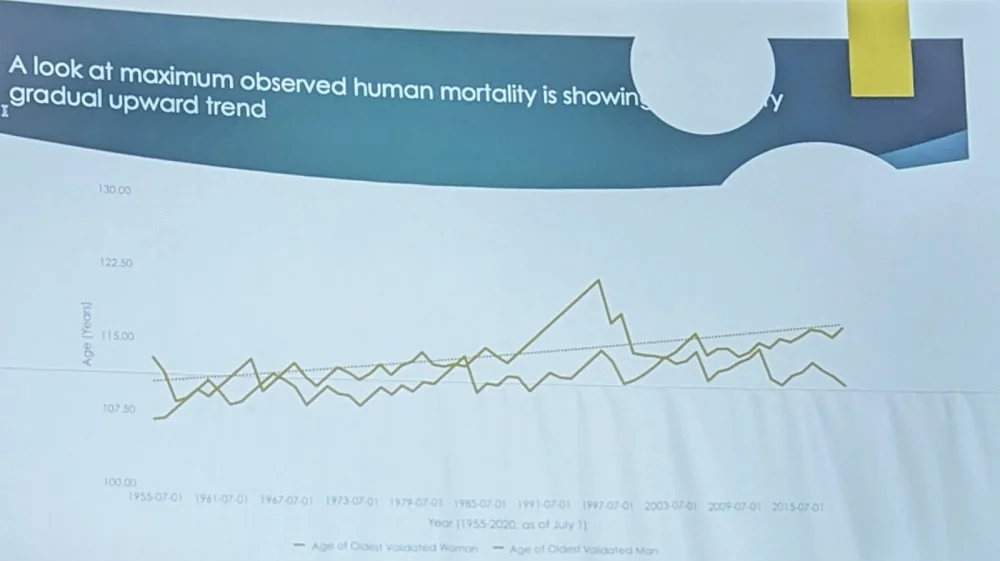
Robert advocated for studying centenarians as the ultimate winners of “the genetic lottery”. This means they had “to get all the numbers right”, so their genome is highly enriched with longevity-enhancing variants.
Interestingly, it looks like centenarians’ causes of death somewhat differ from those of shorter-lived people. For instance, a groundbreaking “supercentenarian autopsy study” from 2012 showed that many of the subjects died from TTR amyloidosis, a disease in which abnormal amyloid deposits are accumulated in several tissues and organs. This condition is barely known in younger people and hence is vastly understudied. Since many more people today are approaching the 100+ age than in earlier cohorts, it might well be that by targeting centenarian-specific diseases, we can break that theoretical 125-year barrier.
Prof. David McCarthy of the University of Georgia is not a biologist. Instead, he specializes in risk management and insurance. What can this statistics-heavy field tell us about maximum human lifespan? Apparently, a lot.
As David explained, it is customary to plot aging trajectories by year of death – that is, scientists look at people who died in a certain year and see the age distribution, including the proportion of centenarians. However, people who died in a given year at the age of 80 (normal human life expectancy) and at the age of 100 belong to different generational cohorts with different lived experiences affecting their lifespan. Older cohorts might have had more exposure to childhood diseases, a less healthy lifestyle, fewer medical advancements, and so on. According to David, when you use year of birth instead of year of death as an explanatory variable, a significant postponement of mortality (as opposed to compression of mortality) can be seen in later cohorts.
However, using the year of birth has its problems, as many people in recent cohorts are still alive, and their mortality patterns at extreme old age cannot be observed. David and his colleagues try to overcome this obstacle by using Bayesian techniques to predict mortality rates for currently living cohorts. While it is hard to say how reliable this method is, according to David, existing data from many countries suggests a shift from compression to true postponement of mortality which is not yet visible in mortality data because people in these cohorts who will reach extreme old age are still alive. Supposedly, this is what creates an illusion of a “hard barrier”. Instead, David optimistically concluded, he expects “longevity records to increase in the coming decades as the cohorts that have enjoyed mortality postponement reach these ages.”
Replace, don’t repair: the Gordian knot approach to aging
Aging is an immensely complex phenomenon. We have only begun to understand it, and there’s still a very long way to fixing it on a cellular level. However, we might be able to take a giant shortcut by replacing aging tissues and organs with young ones. That requires bold thinking and being ready to discuss complex ethical issues.
Justin Rebo, CEO of Kind Biotechnology, delivered a snapshot of the current ideas in the field of replacement, starting with the easiest tasks such as replacing cellular components of the blood: something we are very close to. The next step should be producing young organs. However, roadblocks lie ahead. The supply of human organs is very limited, xenogenic organs are associated with immense rejection problems, and bioprinting is currently not able to produce complex organs, with the possible exception of the liver.
How do you grow a complex organ, if, as Justin put it, “every organ needs every other organ to develop”? The right way to do it, he argued, is by growing them simultaneously. The “problem” is, sets of organs come with a central nervous system attached to them. However, genetic modifications might be able to solve that, giving us a supply of organs that are inherently “orphaned”, i.e., not belonging to a person.
While this might sound unsettling, people in the longevity field assume that extreme circumstances (such as 70-80% of the population dying of aging) call for extreme measures. Any ethical concerns, however valid, should be carefully weighed against the immense amount of suffering and death associated with aging.
There might be easier ways to produce organs, such as bionics. Some organs might not be too complex to manufacture (the heart, for instance, is little more than a pump). Partially successful attempts to build an artificial heart have been going on for decades, but today’s vastly improved technology might finally help scientists crack this problem. Kidneys can already be replaced by a machine, the main problem being miniaturization.
In the second replacement-related talk, Peter Kondaurov, an entrepreneur with little background in biology, delivered a bold vision of full-body replacement pursued by his company Sybody. The company, according to Peter, draws inspiration from unique and bizarre experiments conducted in the Soviet Union half a century ago, when dogs had a second head attached to their bodies. The heads were attached to the vasculature, but not to the spinal cord, making them merely decorations, and the dogs did not survive for long. The experiments were quickly stopped, but Peter is certain that attaching an old head to a young body can work. The question is, of course, where to get the body, and how to rejuvenate brain tissue (something that scientists such as Jean Hebert are working on). Meanwhile, Sybody plans to issue its own crypto token for people who wish to support its work and maybe be the first to have their bodies replaced with younger versions.
Of course, we are aware of the scientific and ethical challenges surrounding some concepts in the longevity space. However, our mission is to faithfully and impartially report on the developments in our field.
Do we need artificial brains to study real ones?
Given the immense complexity of aging, many geroscientists put their hopes in AI’s rapidly growing powers. AI is already being used for drug discovery, building aging clocks, and multiple other purposes. However, the holy grail that is being increasingly discussed is building a foundation model of biology, something that would take our knowledge of how our bodies work to a completely new level. Several startups are currently working in this field, inspired by the immense success of LLMs (large language models) and of biology-specific models such as Google’s AlphaFold.
In his talk, Dmitri Kalupin, founder of Unlock Biology, gave an overview of various possible avenues that this field can pursue. Until now, progress has been bumpy, hampered by insufficient data and resources. A lot has been said about a computational model of a cell, but we are still very far from that. Scarcity of good data means that we need, in Dmitri’s words, “to put biological intuitions into computer models”, and successful projects like AlphaFold are a sign that this can be done.
This field will probably be pushed forward by humanity “investing billions in wet lab experiments” to acquire enough quality data and feeding this data to large-scale computer models. This will result in trillions of dollars in value from digital experiments (simulations) that will at least partially replace the good old wet lab.
Data about the workings of the human body is hidden in various “omics” – genomics that cover genes and their mutations, transcriptomics which tell us how those genes are transcribed into RNA, proteomics which deal with the protein makeup of cells, and so on. Which are most suitable for building large-scale models of biology?
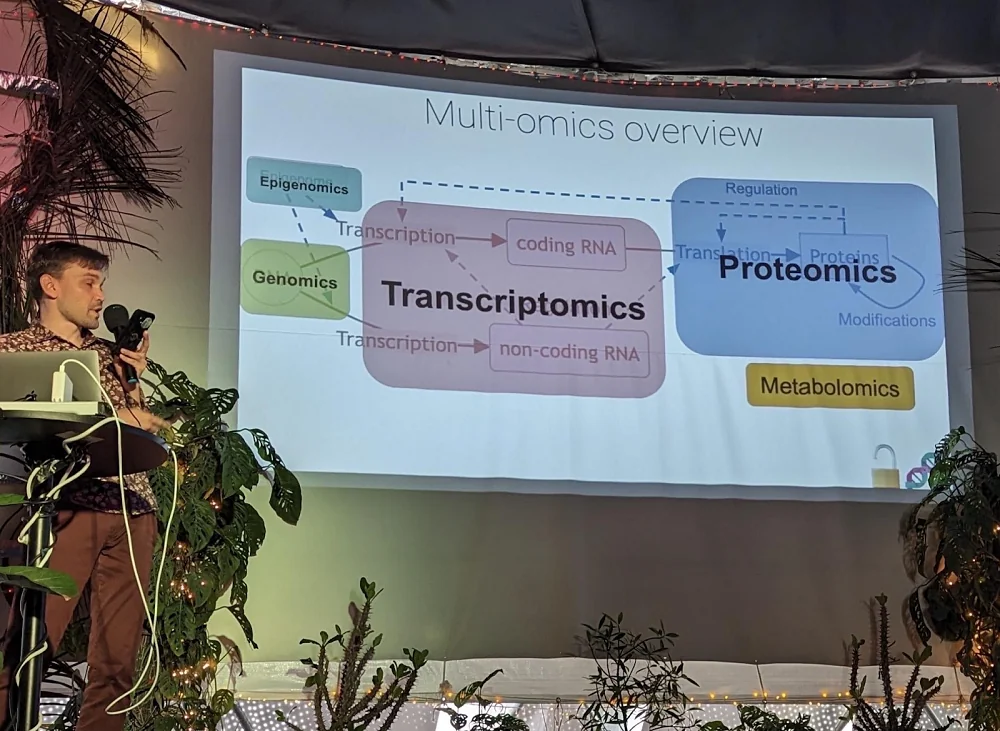
Apparently, all have their pros and cons. For instance, proteomics, Dmitri said, “show the building blocks of cells”, and proteins can be directly modified by clinical interventions, but, on the other hand, they are hard to measure and currently only yield about 500 scientific papers a year, meaning the rate of data acquisition is low.
Dmitri’s choice is transcriptomics. RNA-measuring techniques have been developed at frantic speeds, outpacing Moore’s law. It’s now so cheap and simple that more and more biology papers include transcriptomic analyses, adding to the growing Everest of data. Among the cons is the fact that the RNA lanscape cannot be exactly mapped to the protein landscape because of various post-transcriptional modifications. In fact, there can be little correlation between the two. Still, the pros, in Dmitri’s opinion, outweigh the cons, and we can expect the era of transcriptomic-based foundation biology models.
Michael Antonov is a new face at longevity conferences, but hopefully, this will change soon. Michael was a co-founder and Chief Software Engineer at Oculus, and he is also a longevity enthusiast. After Oculus’ success, Michael founded Deep Origin, a company that, according to its website, is into “empowering deep understanding of biology by building tools that simplify R&D, simulate biology, and untangle the complexity of life.”
In his talk, Michael too stressed the complexity of biology and the urgent need to model it computationally. “What we don’t model, we don’t understand”, he said. While we can understand basic principles, the huge amount of detail must be filled in by predictive models and simulations before we can hope to alter phenotypes towards more youthful states.
Like Dmitri, Michael maintained that there is no substitute for a lot of wet lab work to amass the amount of data needed to model biology. However, biologists should be armed with the best possible software tools, which is one of the things that Deep Origin is working on.
Some of the tools are already accessible via Deep Origin’s website. The computational biology platform is currently in beta mode, aiming to provide scientists with a “cloud lab” for computation, analysis, and collaboration. A molecular simulation toolkit for drug discovery is in the works and will be available soon. It will include virtual screening, molecular dynamics, coarse-grained (simplified) protein folding, pathway analysis, and so on.
Michael then expanded on the topic of biological simulation. Clearly, he said, we cannot simulate a living system “from the atoms up” since there are trillions of atoms in a single cell. However, humanity has had considerable success with simulations of complex systems that we do not understand down to the most fundamental level, such as weather. Michael thinks that we are currently approaching this ability in biology. In several years, we might be able to predict the effect of a novel molecule on a cell completely in silico.