The Fibroblasts That Protect Ovarian Cancer
- These fibroblasts are found guarding many other cancers as well.
In Aging, researchers have described a subpopulation of fibroblasts that nurture ovarian cancer tumors and shield them from harm.
Cancer’s natural defenders
Ovarian cancer is particularly dangerous, both in death rates and in recurrence [1]. Normally, chemicals that contain platinum are effective, but this sort of cancer develops resistance to such treatments; after that, prognoses are usually very poor, even with modern drugs [2].
This cancer’s tumors, however, are not just made up of cancer cells: cancer-associated fibroblasts (CAFs) support its progression [3] and that of other cancers. These cells are in constant intercellular communication with the cancer, sending a steady stream of signals back and forth [4] and remodeling the extracellular matrix (ECM) around the tumor, which makes drug delivery uniquely difficult [5]. Even though the tumor microenvironment is highly inflammatory, which can be used to trigger immunotherapies [6], one particuar compound in these tumors, biglycan, impedes the effectiveness of such therapies [4].
CAFs are heterogenous, coming from multiple sources. Most of them began as resting fibroblasts, but others started out as muscle cells or structural cells of the blood vessels or other organs [7]. These cells do not even share the same differentiation, as some of them have stem cell origins [8].
Prior work has made efforts to describe these subpopulations of cells in order to more precisely target them [9]. Such categorizations include their origins, their development, their inflammatory status and antigens, and how they can be targeted in therapies.
dCAF is not good for you
After finding appropriate biomarkers for identification, this study divided fibroblasts into three of these categories: myofibroblastic (myCAFs), inflammatory (iCAFs), and desmoplastic (dCAFs), the last of which has been associated with poor prognoses. These three different types have very distinct clusters, both in gene expression and in physical distribution.
As expected, myCAFs express genes related to muscle function and iCAFs express inflammatory factors, while dCAFs express collagen-forming genes. While the iCAFs, as expected, communicated quite a bit, predominantly through their inflammatory factors, it was the dCAFs that communicated the most, using the ECM to do so.
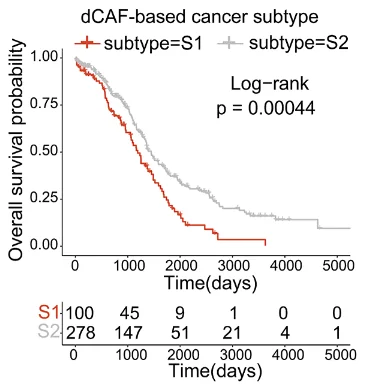
Low-dCAF cancers were found to be far less dangerous than high-dCAF cancers. Genes associated with platinum resistance were much more upregulated in the high-dCAF group, and they showed signs of being resistant to multiple other drugs. As dCAF is correlated with collagen production, it is no surprise that the high-dCAF group’s tumors were richer in collagen, and therefore stiffer and tougher, than the low-dCAF group’s.
The researchers were able to use gene expression analysis to construct a prognostic test through a machine learning algorithm. Patients who had more dCAF genes expressed were, indeed, more likely to die of cancer under modern treatment conditions.
While this paper’s authors did not get into how dCAFs could be specifically targeted and eliminated, they hold that this is a promising therapeutic strategy. If these very particular cells are found to be vulnerable to a novel therapy, the prognosis of people with ovarian cancer or many other cancers may significantly improve.
Literature
[1] Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: a cancer journal for clinicians, 73(1), 17-48.
[2] Gaillard, S., Oaknin, A., Ray-Coquard, I., Vergote, I., Scambia, G., Colombo, N., … & Lorusso, D. (2021). Lurbinectedin versus pegylated liposomal doxorubicin or topotecan in patients with platinum-resistant ovarian cancer: a multicenter, randomized, controlled, open-label phase 3 study (CORAIL). Gynecologic oncology, 163(2), 237-245.
[3] Rimal, R., Desai, P., Daware, R., Hosseinnejad, A., Prakash, J., Lammers, T., & Singh, S. (2022). Cancer-associated fibroblasts: Origin, function, imaging, and therapeutic targeting. Advanced drug delivery reviews, 189, 114504.
[4] Zheng, S., Liang, J. Y., Tang, Y., Xie, J., Zou, Y., Yang, A., … & Lin, Y. (2023). Dissecting the role of cancer‐associated fibroblast‐derived biglycan as a potential therapeutic target in immunotherapy resistance: A tumor bulk and single‐cell transcriptomic study. Clinical and Translational Medicine, 13(2), e1189.
[5] Guo, J., Zeng, H., & Chen, Y. (2020). Emerging nano drug delivery systems targeting cancer-associated fibroblasts for improved antitumor effect and tumor drug penetration. Molecular pharmaceutics, 17(4), 1028-1048.
[6] Feig, C., Jones, J. O., Kraman, M., Wells, R. J., Deonarine, A., Chan, D. S., … & Fearon, D. T. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences, 110(50), 20212-20217.
[7] Huang, J., Tsang, W. Y., Li, Z. H., & Guan, X. Y. (2023). The origin, differentiation, and functions of cancer-associated fibroblasts in gastrointestinal cancer. Cellular and Molecular Gastroenterology and Hepatology.
[8] Kanzaki, R., & Pietras, K. (2020). Heterogeneity of cancer‐associated fibroblasts: opportunities for precision medicine. Cancer science, 111(8), 2708-2717.
[9] Öhlund, D., Handly-Santana, A., Biffi, G., Elyada, E., Almeida, A. S., Ponz-Sarvise, M., … & Tuveson, D. A. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. Journal of Experimental Medicine, 214(3), 579-596.







