Rejuvenating the Hippocampus With Metabolites
- This approach may be more feasible than others.
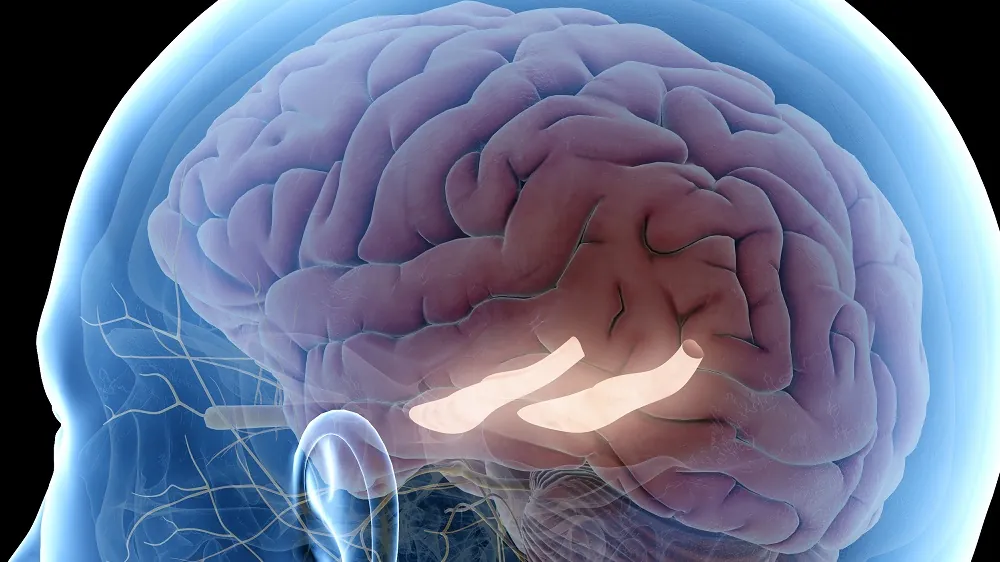
Researchers have reported in Aging Cell that injecting specific one-carbon metabolites into the hippocampus can rejuvenate its cells.
A small molecule approach

Read More
Epigenetic alterations have been found to impact the neuroplasticity of the brain, leading to problems with learning and memory [1]. This research team has previously found that reversing these alterations with Yamanaka factors has positive effects on memory retention, restoring it to a more youthful state [2]. However, these researchers contend that approaches using these factors are still out of reach for clinical use.
Instead, they focus on metabolites derived from one-carbon (1C) pathways, which are involved in the basics of epigenetic methylation. Previous work has found that exposing cells to these metabolites in a 1C-MIM cocktail results in epigenetic rejuvenation, restoring function to brain astrocytes and muscle strength to older mice [3]. However, two components of that cocktail were found to be harmful to neurons.
Effects on epigenetics and gene expression
These researchers decided to omit those two compounds, creating 1C-4-MIM. Because memory loss is prominent in aging, the researchers injected this reduced cocktail directly into the dentate gyrus, the part of the hippocampus most responsible for memory formation, in 12-month-old mice. They found that H4K20me3, an epigenetic marker that increases with age, was reduced in these mice; however, H3K9me3, which decreases with age, was unaffected.
Testing further on 4-month-old and 16-month-old mice, the researchers did not find evidence that a single dose of these metabolites reduces epigenetic age acceleration. However, 248 separate epigenetic loci were found to be significantly affected by the treatment. The researchers found that the two that were most affected are already known to have effects on the brain: Cacna2d2 controls calcium channels related to plasticity, while Zic4 affects a transcriptor associated with both memory and spatial learning [4].
Several other genes related to memory and learning were also affected, and, unsurprisingly, many more genes were significantly affected in older mice than younger mice. These results were replicated in RNA sequencing, which found that genes related to cellular proliferation were also affected; indeed, both proliferation in cells and the formation of new neurons (neurogenesis) in the mice were improved, as was the neuroplasticity marker GluN2B. Some of these effects may have been related to changes in oxidative stress, although the researchers could not confirm this finding.
Works on mice, but does it work on other animals?
Most importantly, these brain changes were found to have concrete effects on the mice’s behavior. Mice that had been injected with 1C-4-MM performed better in both a maze-based memory test and an object recognition test.
While it is unclear if this cocktail is safe or effective for human use, and this cocktail was not tested on truly old (22- to 24–month) mice, these are promising results that warrant further experimentation, possibly with a careful analysis of oxidative stress and with different species.
Literature
[1] Creighton, S. D., Stefanelli, G., Reda, A., & Zovkic, I. B. (2020). Epigenetic mechanisms of learning and memory: implications for aging. International Journal of Molecular Sciences, 21(18), 6918.
[2] Rodríguez-Matellán, A., Alcazar, N., Hernández, F., Serrano, M., & Ávila, J. (2020). In vivo reprogramming ameliorates aging features in dentate gyrus cells and improves memory in mice. Stem Cell Reports, 15(5), 1056-1066.
[3] Hernandez-Benitez, R., Wang, C., Shi, L., Ouchi, Y., Zhong, C., Hishida, T., … & Belmonte, J. C. I. (2024). Intervention with metabolites emulating endogenous cell transitions accelerates muscle regeneration in young and aged mice. Cell Reports Medicine, 5(3).
[4] Chiavellini, P., Lehmann, M., Canatelli Mallat, M., Zoller, J. A., Herenu, C. B., Morel, G. R., … & Goya, R. G. (2022). Hippocampal DNA methylation, epigenetic age, and spatial memory performance in young and old rats. The Journals of Gerontology: Series A, 77(12), 2387-2394.