Generating Neural Progenitor Cells from iPSCs

The latest research published in the Journal of Tissue Engineering and Regenerative Medicine has reprogrammed pig fibroblast cells into neural progenitor cells.
Manufacturing Pig Brain Cells
Brain regeneration is a novel and potentially groundbreaking strategy for improving brain health. Researchers have used these techniques both to stimulate the body’s own regeneration capabilities and to create miniature brain organoids, which can be used to study the pathology of neural tissues. However, unlike similar strategies applied to other tissues, the cells of neural tissues are especially difficult to work with. A biopsy cannot easily be taken from the brain like it can with bone marrow or skin. Furthermore, neural stem cells are notoriously difficult to culture and expand, even after isolation from a biopsy sample.
However, cellular reprogramming can take more common cell types, such as fibroblasts, and convert them to pluripotent stem cells using the Yamanaka factors (OCT4, SOX2, KLF4, and c-Myc). Recently, researchers at the University of São Paulo have used this technique in pigs to create induced pluripotent stem cells (iPSCs) and then differentiate them to neural progenitor cells. While this has been done in many studies using human and mouse cells, this process has been rarely studied in pigs, despite them being important both agriculturally and as preclinical models.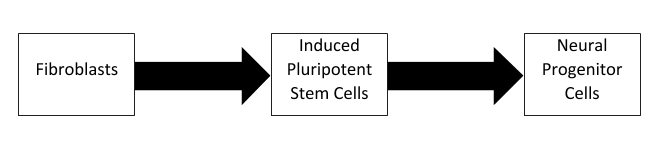 The researchers optimized their technique and characterized the resulting iPSCs and neural progenitor cells. Both their reprogramming and differentiation was considered successful based on identifying markers of pluripotent stem cells, such as OCT4 and NANOG, and neural progenitor cells, such as Nestin and GFAP. This success means that other researchers can confidently utilize their methods for future porcine research. Furthermore, the research identified several differences between pigs and humans, potentially shedding light on the underlying physiological differences involved.
The researchers optimized their technique and characterized the resulting iPSCs and neural progenitor cells. Both their reprogramming and differentiation was considered successful based on identifying markers of pluripotent stem cells, such as OCT4 and NANOG, and neural progenitor cells, such as Nestin and GFAP. This success means that other researchers can confidently utilize their methods for future porcine research. Furthermore, the research identified several differences between pigs and humans, potentially shedding light on the underlying physiological differences involved.
In this study, we were able to induce porcine embryonic fibroblasts to a pluripotentlike state. Generated piPSC exhibited the expected morphology, expressed endogenous pluripotency genes (pOCT4 and pNANOG), and were AP-, OCT4-, NANOG-, SSEA1-, and TRA1-60-positive, as detected by immunostaining; however, the three clonal colonies showed diverse expression profiles. Aiming to derive NPCs, two lines of piPSC were subjected to a neuronal induction protocol, and the obtained cells expressed pNestin and pGFAP and were positively stained for Nestin, β Tubulin III, and Vimentin, suggesting a commitment to neural differentiation. Further studies aiming to generate fully reprogrammed footprint-free piPSCs that are more suitable for specific cell-line differentiation are of great importance for better understanding, reprogramming, and characterization of porcine NPCs.
Conclusion
Pig physiology is surprisingly similar to that of humans, making swine a valuable preclinical model. While this is not a direct brain regeneration technique, the methods presented in this study can help researchers on their quest to better understand brain physiology, pathology, and neuroregeneration.