Why NAD+ Declines With Age

Nicotinamide adenine dinucleotide (NAD+), a nucleotide, is critical for life to exist. From the most simple bacteria to complex multicellular organisms such as humans, NAD is a vital component of cellular function and thus life.
An increased level of NAD+ appears to convey health and longevity, and a decrease is associated with aging and disease. Today, we are going to look at NAD+, why it declines with age, and what science might do about it.
What is NAD?
NAD+ is abundantly found in the majority of living cells and is involved in electron transfer and the regulation of various biological pathways, from intracellular calcium transients to the epigenetic status of chromatin. NAD+ provides a critical link between cellular signalling and metabolism, and it is a key player in the metabolic nutrient sensing pathways.
NAD is best known for its role as a coenzyme involved in redox reactions, transporting electrons from one reaction to another within the cell, and linking the catabolic reactions of glycolysis and the citric acid cycle to oxidative phosphorylation.
However, in the last twenty years, NAD+ has been discovered to also play a role as a signalling molecule. In all species, increased levels of NAD+ cause cells to make changes that improve their survival; this includes increased energy production and use, improved cellular repair, and coordinating circadian rhythms.
NAD+ is converted into signals by various enzymes that are designed to sense NAD+, including the sirtuins (SIRT1–SIRT7), CtBP1 and 2, and poly-ADP-ribose polymerases(PARPs). Recent studies have shown that mice treated with PARP inhibitors or NAD+ precursors have health benefits. Some of the observed benefits have been increased insulin sensitivity, reduced mitochondrial dysfunction, reduced cellular senescence, and increased lifespan [1-3]. Unfortunately, with age, levels of NAD+ fall in all studied species.
NAD+ declines with age because it is destroyed
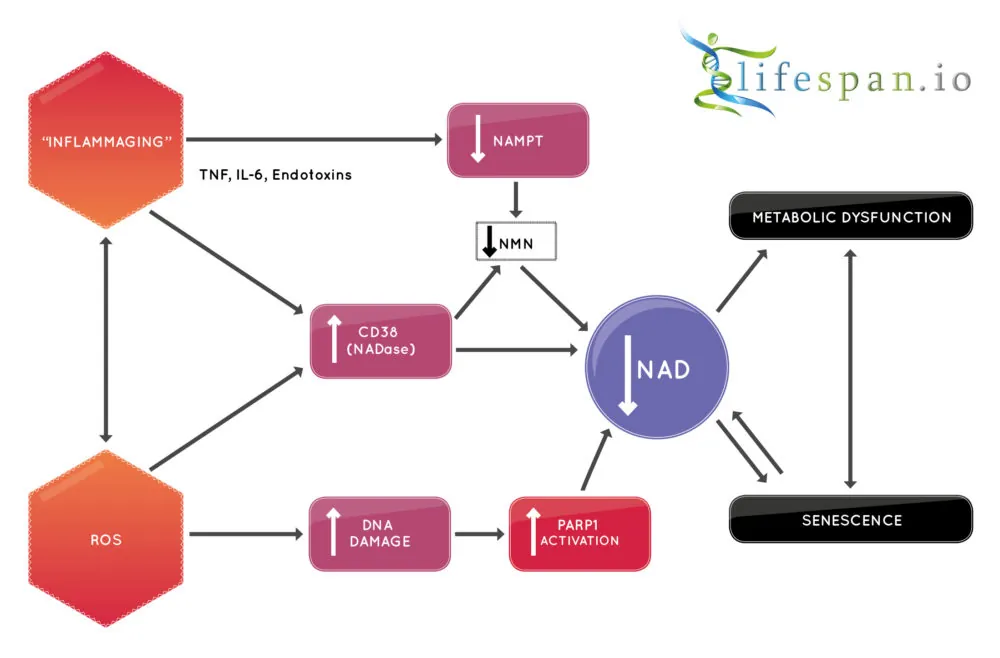
It has been known for some time that NAD levels decline during the aging process, but it is actively destroyed by the enzyme CD38 [4-5]. CD38 is a membrane-bound NADase that hydrolyzes NAD+ to nicotinamide and (cyclic-)ADP-ribose. It is associated with immune responses and energy metabolism, but it is also a NADase whose levels rise with aging, with a corresponding increase in NADase activity and a decrease of NAD+.
Tests have shown that mice bred to be deficient in CD38 enjoy increased protection from mitochondrial dysfunction and are resistant to diabetes as they age. This protective action is regulated via the mitochondrial sirtuin SIRT3. Research shows that mice treated with the CD38 inhibitor apigenin, a flavone found in many plants, show increased levels of NAD+ and are resistant to the effects of high-fat diets [6].
A 2016 study showed that protein levels of CD38 increase in multiple tissues during the aging process, with a corresponding rise in CD38 activity [7]. One of the hallmarks of aging is that of mitochondrial dysfunction, and this study showed that cells with high levels of CD38 use less oxygen, have increased lactate, and have dysfunctional mitochondria. In the mitochondria of the livers of CD38 knockout mice, more oxygen is consumed, and they have greater mitochondrial membrane potential.
The current approach to address the loss of NAD+ is to increase it with NAD+ precursors, such as niacin, nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN). As CD38 actively degrades both NAD+ and NMN, it may be a useful approach to combine such NAD+ boosting therapies with CD38 inhibitors to increase potency.
Inflammaging likely drives NAD+ depletion in aging
We now know that CD38 is the major culprit in NAD+ decline, but why does it fall in the first place? CD38 expression and activity are known to be induced by inflammatory cytokines and bacterial endotoxins, such as lipopolysaccharides (LPS) [8-11]. This strongly suggests that the smouldering, age-related, chronic inflammation commonly called “inflammaging” may be driving the increased expression of CD38 and the resulting NAD+ decline.
This is because senescent cells secrete CD38 as part of the pro-inflammatory cytokine cocktail known as the senescence-associated secretory phenotype (SASP). This mixture of pro-inflammatory signals includes CD38, and senescent cells accumulate during aging as the immune system increasingly fails to remove the problem cells. Thus, more senescent cells almost certainly means more CD38 and less NAD+ available.
The same goes for increasing amounts of cell debris and microbial burden; these also drive inflammaging, thus increasing CD38, decreasing NAD+, and causing age-related dysfunction.
 Therapies that increase NAD+ in cells, and potentially co-therapies that reduce CD38, are a potential way to slow an aging process and may help to treat metabolic disorders such as diabetes. There is potential for such approaches to increase the number of years we spend healthy, and it may even increase lifespan as it does in other species; the good news is that we may soon find out.
Therapies that increase NAD+ in cells, and potentially co-therapies that reduce CD38, are a potential way to slow an aging process and may help to treat metabolic disorders such as diabetes. There is potential for such approaches to increase the number of years we spend healthy, and it may even increase lifespan as it does in other species; the good news is that we may soon find out.
Conclusion
NAD+ boosting therapies represent a near-future prospect, given that they are currently already in small-scale human trials right now, with a view to moving to larger human studies in the future. This therapy can be considered a true rejuvenation therapy, as it directly addresses the aging hallmark of deregulated nutrient sensing and partially addresses genomic instability via encouraging DNA repair. It will be interesting to see if this or senescent cell clearance will be the next rejuvenation technology to arrive, given that stem cell therapy is already here.
Readers may also be interested to learn that the David Sinclair lab at Harvard Medical School has also successfully launched the NAD+ Mouse Project on Lifespan.io, this project aims to test NAD+ replacement therapies on mice and study the long-term effects on health and lifespan.
Literature
[1] Bai, P., Cantó, C., Oudart, H., Brunyánszki, A., Cen, Y., Thomas, C., … & Schoonjans, K. (2011). PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell metabolism, 13(4), 461-468.
[2] Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., … & Mercken, E. M. (2013). Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624-1638.
[3] Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., … & Schoonjans, K. (2016). NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science, 352(6292), 1436-1443.
[4] Camacho-Pereira, J., Tarragó, M. G., Chini, C. C., Nin, V., Escande, C., Warner, G. M., … & Chini, E. N. (2016). CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell metabolism, 23(6), 1127-1139.
[5] Schultz, M. B., & Sinclair, D. A. (2016). Why NAD+ declines during aging: It’s destroyed. Cell metabolism, 23(6), 965-966.
[6] Escande, C., Nin, V., Price, N. L., Capellini, V., Gomes, A. P., Barbosa, M. T., … & Chini, E. N. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes, 62(4), 1084-1093.
[7] Camacho-Pereira, J., Tarragó, M. G., Chini, C. C., Nin, V., Escande, C., Warner, G. M., … & Chini, E. N. (2016). CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell metabolism, 23(6), 1127-1139.
[8] Lee, C. U., Song, E. K., Yoo, C. H., Kwak, Y. K., & Han, M. K. (2012). Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Molecules and cells, 34(6), 573-576.
[9] Musso, T., Deaglio, S., Franco, L., Calosso, L., Badolato, R., Garbarino, G., … & Malavasi, F. (2001). CD38 expression and functional activities are up‐regulated by IFN‐γ on human monocytes and monocytic cell lines. Journal of leukocyte biology, 69(4), 605-612.
[10] Sun, L., Iqbal, J., Zaidi, S., Zhu, L. L., Zhang, X., Peng, Y., … & Zaidi, M. (2006). Structure and functional regulation of the CD38 promoter. Biochemical and biophysical research communications, 341(3), 804-809.
[11] Yamamoto-Katayama, S., Ariyoshi, M., Ishihara, K., Hirano, T., Jingami, H., & Morikawa, K. (2002). Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities1. Journal of molecular biology, 316(3), 711-723.








