NAD+ and Cellular Senescence Pathways Interact

A new publication highlights how the complex interaction of NAD+ and cellular senescence pathways may complicate proposed anti-aging therapies that boost NAD+ using precursors.
What are epigenetic alterations?
One of the proposed reasons we age is the changes to gene expression that our cells experience as we get older; these are commonly called epigenetic alterations. These alterations harm the fundamental functions of our cells and can increase the risk of cancer and other age-related diseases.
The DNA in each of our cells is the same, with only slight differences, so why do our various organs and tissues look so different, and how do cells know what to become?Gene expression is modified by the addition of epigenetic markers to the DNA changing the pattern of gene expression in a cell, suppressing or enhancing the expression of certain genes in a cell as the situation demands. You might think of DNA as the building blocks and epigenetics as the instruction manual that explains how to assemble those blocks to make a certain structure to suit a particular situation.
This is how a cell in the liver knows that it needs to be a liver cell: the epigenetic instructions make sure that it is given the right guidance to become the correct cell type. At a basic level, these epigenetic instructions make sure that the genes needed to develop into a liver cell are turned on while the instructions specific to other types of cells are turned off.
However, as we age, our cells are exposed to environmental factors and are subject to negative changes in their genome through epigenetic mechanisms. Such changes accumulate over time and have been correlated with the decline observed in aging cells.
Epigenetic alterations in aging include changes to methylation patterns and in general, these correlate with a decrease in the amount of heterochromatin and an increase in chromosome fragility and transcriptional alterations (variance in gene expression), remodeling of chromatin (a DNA support structure that assists or impedes its transcription), and transcriptional noise.
The consequences of epigenetic alterations
If epigenetic alterations happen, then the cell could potentially lose its cell memory, forgetting what type of cell it is, or begin to behave in a harmful manner. This appears to be the case with the thymus; when we age, the thymic epithelial cells of that organ start to lose cell identity and begin to change into fat or mesenchymal cells.
Another example of how epigenetic alterations can harm us is the immune system; changes to cell behavior there can interfere with immune cell activation or suppress immune cells, leaving us vulnerable to pathogens.
One consequence of this is the inappropriate activation of the immune system, which causes inflammation and contributes to the age-related background of chronic inflammation. This is known as “inflammaging“, and it harms tissue repair and prevents effective regeneration. Metabolism and epigenetic alterations are closely linked with inflammation, facilitating a feedback loop that leads to ever-worsening epigenetic alterations.
Partial cellular reprogramming appears to be a potential way to fix this aging process and reset the cells to a younger epigenetic state.
Interacting NAD+ and cellular senescence pathways
Essentially, age-related epigenetic alterations can cause cells to become functionally compromised or less efficient as well as potentially dysfunctional. The weight of evidence is increasingly supporting the primary role that epigenetic alterations have during aging and how they drive and interact with the other aging processes.
Two age-related changes driven at least in part by epigenetic alterations that are demonstrably treatable are the accumulation of senescent cells and the decline of NAD+ levels in cells; however, while it is clear that the two are linked, the exact relationship between these two has been unclear.
NAD+
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. It is a dinucleotide, which means that it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide.
NAD+ is created from simple building blocks, such as the amino acid tryptophan, and it is created in a more complex way via the intake of food that contains nicotinic acid (niacin) or other NAD+ precursors. These different pathways ultimately feed into a salvage pathway, which recycles them back into the active NAD+ form.
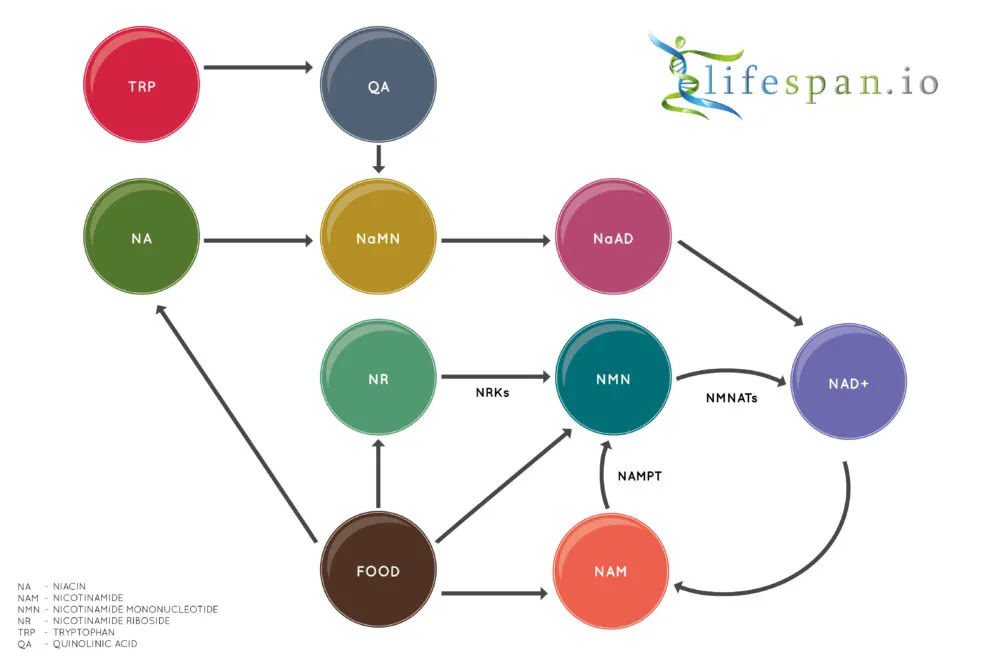
In metabolism, NAD facilitates redox reactions, carrying electrons from one reaction to another. This means that NAD is found in two forms in the cell: NAD+ is an oxidizing agent that takes electrons from other molecules in order to become its reduced form, NADH. NADH can then become a reducing agent that donates the electrons it carries. The transfer of electrons is one of the main functions of NAD, though it also performs other cellular processes, including acting as a substrate for enzymes that add or remove chemical groups from proteins in post-translational modifications.
NAD plays a central role in metabolism and cell signaling and function facilitating glycolysis, oxidative phosphorylation, the citric acid cycle, and poly-ADP-ribose polymerases (PARPs), sirtuins, and CD38/157 ectoenzymes. NAD facilitates DNA repair via its interaction with PARPs as well as interacting with sirtuins, which are protein deacetylases that regulate cell survival, cell cycle, apoptosis, mitochondrial function, and energy production.
Accumulating evidence suggests that NAD+ systemically declines with age in a variety of organisms, including rodents and humans, which contributes to the development of many age-related diseases.
Quite simply, without NAD, life would be impossible, so it is unsurprising that the decline of NAD is linked to the various proposed causes of aging: DNA damage, mitochondrial dysfunction, loss of proteostasis, deregulated nutrient sensing, stem cell exhaustion, and epigenetic alterations. For this reason, there is a great deal of interest in boosting NAD+ levels via precursors, including nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), with the hope of delaying or even preventing certain aspects of age-related functional decline and diseases.
Cellular senescence
As we age, increasing numbers of our cells enter into a state known as cellular senescence, which, like epigenetic alterations, is one of the hallmarks of aging.
Senescent cells do not divide or support the tissues of which they are part; instead, they emit a range of potentially harmful chemical signals that encourage nearby healthy cells to enter the same senescent state. Their presence causes many problems: they reduce tissue repair, increase chronic inflammation, and can even eventually raise the risk of cancer and other age-related diseases.
Senescent cells normally destroy themselves via a programmed process called apoptosis, and they are also removed by the immune system; however, the immune system weakens with age, and increasing numbers of senescent cells escape this process and begin to accumulate in all the tissues of the body.Senescent cells only make up a small number of total cells in the body, but they secrete pro-inflammatory cytokines, chemokines, and extracellular matrix proteases, which, together, form the senescence-associated secretory phenotype, or SASP.
By the time people reach old age, significant numbers of these senescent cells have built up, causing chronic inflammation and damage to surrounding cells and tissues via the SASP.
The SASP is thought to significantly contribute to aging and cancer; thus, targeting senescent cells and removing them using senolytic drugs has been suggested as a potential solution to this problem.
NAD and cellular senescence pathways collide
One potential hurdle with NAD-boosting therapies is coming to light, however. The NAD+ and SASP pathways interact with each other, and this is becoming clearer as more research data arrives. The new publication highlights how these two pathways interact and how NAD levels are directly related to the expression of the SASP, which may confound attempts to boost NAD using precursors as an anti-aging therapy [2]. The paper reviews the various studies and takes a look at NAD+ and cancer risk as well as why increasing NAD+ levels using precursors may be a trade-off.
However, perhaps the most interesting part of this paper is the suggestion that senolytics may eliminate the need for NAD+ boosting therapies entirely in the context of anti-aging. This actually makes a great deal of sense.
We talked about how CD38 expression destroys NAD in a previous article and that CD38 is part of the inflammatory SASP, which means that the more senescent cells there are, the more CD38 there is, which leads to less NAD. Therefore, removing senescent cells using senolytics should reduce CD38 and lead to a corresponding increase in NAD; this was certainly shown when CD38 was inhibited using quercetin and apigenin in a 2013 experiment [3].
During human aging, decrease of NAD+ levels is associated with potentially reversible dysfunction in the liver, kidney, skeletal and cardiac muscle, endothelial cells and neurons. At the same time, the number of senescent cells, associated with damage or stress that secrete pro-inflammatory factors (SASP, Senescence-Associated Secretory Phenotype), increases with age in many key tissues, including the kidneys, lungs, blood vessels, and brain. Senescent cells are believed to contribute to numerous age-associated pathologies and their elimination by senolytic regimens appears to help in numerous preclinical aging associated disease models including those for atherosclerosis, idiopathic pulmonary fibrosis, diabetes, and osteoarthritis.
A recent report links these processes, such that decreased NAD+ levels associated with aging may attenuate the SASP phenotype potentially reducing its pathological effect. Conversely increasing NAD+ levels by supplementation or genetic manipulation which may benefit tissue homeostasis, also may worsen SASP and encourage tumorigenesis at least in mouse models of cancer. Taken together these findings suggest a fundamental trade-off in treating aging related diseases with drugs or supplements that increase NAD+.
Even more interesting is a report that senescent cells can induce CD38 on macrophages and endothelial cells. In turn increased CD38 expression is believed to be the key modulator of lowered NAD+ levels with aging in mammals. So accumulation of senescent cells may itself be a root cause of decreased NAD+, which in turn could promote dysfunction. On the other hand, the lower NAD+ levels may attenuate SASP, decreasing the pathological influence of senescence. The elimination of most senescent cells by senolysis before initiating NAD+ therapies may be beneficial and increase safety, and in the best case scenario even eliminate the need for NAD+ supplementation.
Conclusion
The interaction of metabolism is complex, and it appears that careful adjustment of NAD+ levels may be required in order for it to be beneficial rather than harmful. Trying to raise NAD+ with precursors may come with too many downsides, preventing us from both having our cake and eating it; alternatively, researchers may find a way to bypass this issue. Only time and research will tell.
It may be the case that in order to restore NAD+ effectively, we must address the chronic inflammation caused by senescent cell accumulation and other sources of inflammaging. Ultimately, we may need partial cellular reprogramming to reset epigenetic alterations and senolytics to remove senescent cells.
Literature
[1] López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217.
[2] Mendelsohn, A. R., & Larrick, J. (2019). Interacting NAD+ and Cell Senescence pathways complicate anti-aging therapies. Rejuvenation research, (ja).
[3] Escande, C., Nin, V., Price, N. L., Capellini, V., Gomes, A. P., Barbosa, M. T., … & Chini, E. N. (2013). Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes, 62(4), 1084-1093.








