A New Approach to NAD+ in Sarcopenia
- This approach targets a different part of the metabolic chain.

Researchers have published a potential new method of treating the age-related muscle loss known as sarcopenia in Aging Cell.
A return to NAD+
Read More
We have previously published information relating to exercise and NAD+ levels, and previous work has investigated the relationship between NAD+ and sarcopenia, the crippling loss of muscle that is all too common in older people [1]. Unsurprisingly, this disorder is closely tied to metabolic dysfunction [2], of which NAD+ plays a large part.
However, there are still no approved drugs for the treatment of sarcopenia, and the effectiveness of established dietary and exercise interventions is limited [3]. Therefore, instead of focusing on NAD+ precursors, such as NMN and NR, this work focuses on a related compound, nicotinamide N-methyltransferase (NNMT), which affects the methylation of both nicotinamide and DNA [4].
A clear biomarker
This experiment began with a gene expression analysis of muscle samples taken from older and younger people. The differentially expressed genes were also related to fundamental metabolic pathways, including AMPK and lipid metabolism. These genes had also been previously implicated in fatty liver disease, diabetes, and obesity.
One of the core genes that was strongly upregulated with sarcopenia was NNMT, which was also found to be significantly upregulated in older muscle compared to young muscle. Sarcopenia-related gene expression changes had significant overlap with the age-related gene expression changes, including fundamental aspects of metabolism and NAD+ processing.
NNMT was least expressed in young people, more expressed in older people, and even more expressed in older people with sarcopenia. Analyzing a previous dataset found that NMN supplementation decreased NNMT expression in older Black 6 mice.
A potential for treatment
With NNMT’s status as a biomarker confirmed, these researchers decided to target it directly. Using a mouse model of accelerated aging using D-galactose for five weeks, they found that the artificially aged mice, as expected, had reduced grip strength; however, this loss of strength was partially ameliorated with NNMT inhibition over this time period, which also rescued some of the muscle size lost to D-galactose.
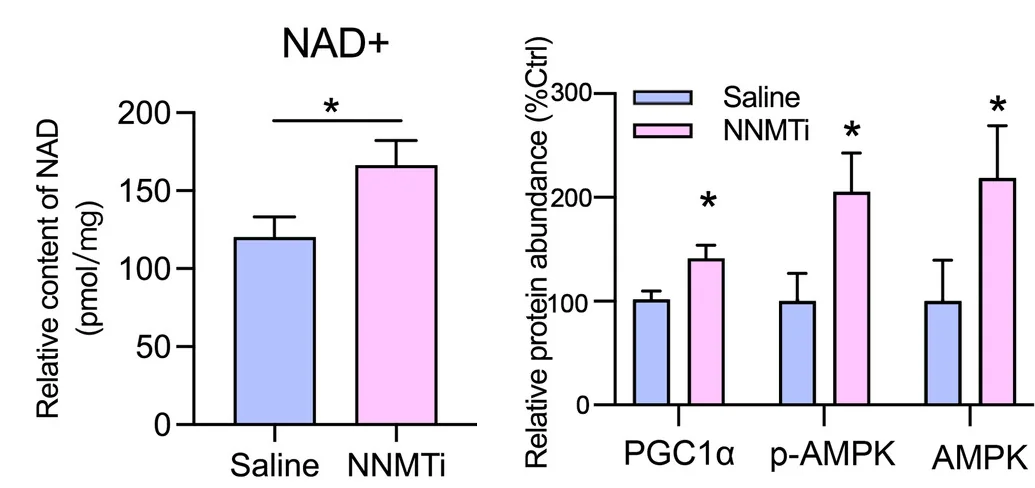
Encouraged, the researchers then turned to naturally aged mice. 19-month-old Black 6 mice were given an NNMT inhibitor for five weeks and compared to a similarly aged control group. The results were very encouraging: their age-related loss of lean mass was attentulated, their grips under electrical stimulation were considerably stronger, metabolic factors such as AMPK and NAD+ were considerably greater, and their cross-sectional muscle mass was larger.
While it is not yet clear what treatment to reduce NNMT could be used for human beings, this metabolic compound is certainly a clear target for interventions. If the metabolic problems underlying sarcopenia could be attenuated through a small molecule or gene therapy, a great many older people would be better protected from this serious disorder and its accompanying loss of independence and lifespan.
Literature
[1] Cruz-Jentoft, A. J., Baeyens, J. P., Bauer, J. M., Boirie, Y., Cederholm, T., Landi, F., … & Zamboni, M. (2010). Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing, 39(4), 412-423.
[2] Daily, J. W., & Park, S. (2022). Sarcopenia is a cause and consequence of metabolic dysregulation in aging humans: effects of gut dysbiosis, glucose dysregulation, diet and lifestyle. Cells, 11(3), 338.
[3] Mellen, R. H., Girotto, O. S., Marques, E. B., Laurindo, L. F., Grippa, P. C., Mendes, C. G., … & Quesada, K. (2023). Insights into pathogenesis, nutritional and drug approach in sarcopenia: a systematic review. Biomedicines, 11(1), 136.
[4] Roberti, A., Fernández, A. F., & Fraga, M. F. (2021). Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Molecular Metabolism, 45, 101165.







