A Strong Link Between Alzheimer’s and Senescence

In a recent study, researchers from the Buck Institute have shown that cellular senescence, one of the hallmarks of aging, is partially responsible for Alzheimer’s disease.

Read More
Cellular senescence is unusual in the brain. As the researchers explain, the normal method for determining whether or not a cell is senescent is to prove that it is no longer capable of cellular division. However, mature neurons do not divide at all, whether senescent or not. Therefore, the researchers of this study propose a unique method of assessing neuronal senescence:
1. Multiple senescence markers need to be used to assess senescence in neurons; 2. The mechanism of action of any identified senescence-inducing stressor should be consistent with that in mitotically-competent cells; and 3. The phenotype should still persist after the senescence-inducing stressor has been removed.
Proteostasis
Through a complicated manufacturing process, our cells use DNA as the template to create proteins, the basic parts that allow our cells to fulfill all of their functions.
Sometimes, however, these proteins do not fold properly. This can be caused by a failure of chaperones that promote proper protein formation, and the resulting misfolded proteins are not always properly destroyed in the lysosomes, the cellular organelles responsible for cleaning up unwanted intracellular debris.
The main pathology of Alzheimer’s disease is that misfolded tau proteins within the cell, along with amyloid beta proteins outside the cell, clump together and form aggregates that clog and destroy neurons. As the researchers of this study explain, one of the most recent hypotheses is that soluble amyloid beta oligomers (AßO), precursors of amyloid beta aggregates, are primarily responsible for Alzheimer’s.
The role of senescence
In this study, the researchers propose that cellular senescence is a major part of the Alzheimer’s cascade. They hold that amyloid beta oligomers promote senescence, directly leading to accumulation of the SASP in the aging brain, which then causes other neurons to become senescent as well. The researchers claim that this cascade, rather than the conventionally understood cascade of amyloid beta aggregation, may be responsible for much of the brain cell loss associated with Alzheimer’s. They also hold that tau pathology itself, rather than being a direct result of Alzheimer’s, may actually be caused by cellular senescence and that immune response may also play a significant role in Alzheimer’s pathology, particularly when the blood-brain barrier is leaky.
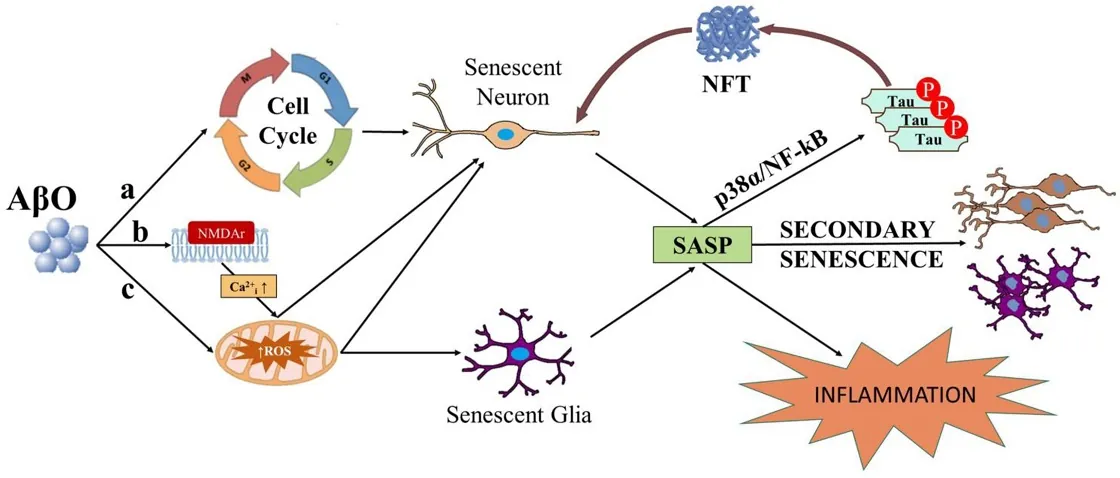
Due to their postmitotic status, the potential for neurons to undergo senescence has historically received little attention. This lack of attention has extended to some non-postmitotic cells as well. Recently, the study of senescence within the central nervous system (CNS) has begun to emerge as a new etiological framework for neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). The presence of senescent cells is known to be deleterious to non-senescent neighboring cells via development of a senescence-associated secretory phenotype (SASP) which includes the release of inflammatory, oxidative, mitogenic, and matrix-degrading factors. Senescence and the SASP have recently been hailed as an alternative to the amyloid cascade hypothesis and the selective killing of senescence cells by senolytic drugs as a substitute for amyloid beta (Aß) targeting antibodies. Here we call for caution in rejecting the amyloid cascade hypothesis and to the dismissal of Aß antibody intervention at least in early disease stages, as Aß oligomers (AßO), and cellular senescence may be inextricably linked. We will review literature that portrays AßO as a stressor capable of inducing senescence. We will discuss research on the potential role of secondary senescence, a process by which senescent cells induce senescence in neighboring cells, in disease progression. Once this seed of senescent cells is present, the elimination of senescence-inducing stressors like Aß would likely be ineffective in abrogating the spread of senescence. This has potential implications for when and why AßO clearance may or may not be effective as a therapeutic for AD. The selective killing of senescent cells by the immune system via immune surveillance naturally curtails the SASP and secondary senescence outside the CNS. Immune privilege restricts the access of peripheral immune cells to the brain parenchyma, making the brain a safe harbor for the spread of senescence and the SASP. However, an increasingly leaky blood brain barrier (BBB) compromises immune privilege in aging AD patients, potentially enabling immune infiltration that could have detrimental consequences in later AD stages. Rather than an alternative etiology, senescence itself may constitute an essential component of the cascade in the amyloid cascade hypothesis.
Conclusion
This study, like many before it, shows that biology is a complicated set of interactions and that even a proteostasis-based disease such as Alzheimer’s may not solely stem from a single hallmark of aging.
Most importantly, however, it provides an explanation for the failure, or very limited success, of treatments that solely focus on amyloid. If the researchers are correct in that cellular senescence is responsible for, or is a major part of, the Alzheimer’s cascade that leads to dementia and death, a combination therapy of senescent cell removal (whether through senolytics or some other method) and treating tau and amyloid pathology at its root may be effective against Alzheimer’s disease.







