Nicotinamide Adenine Dinucleotide (NAD): Benefits and Research
What is NAD+?
Nicotinamide adenine dinucleotide (NAD+) is a key coenzyme found in all living cells. It is a dinucleotide, which means that it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide.
NAD+ is essential for life, one of the most versatile molecules in the body, and an important area of focus for aging research. Before we dive into the various things NAD+ does in the body, let’s take a quick look at its history.
History of NAD+
Back in 1906, William John Young and Arthur Harden discovered there was something in liquid extracted from brewing yeast that could boost the fermentation of sugar into alcohol. At the time, this special something was known as a “coferment” due to this property, but we know it today as NAD+.
Harden continued to explore the science of fermentation and joined forces with Hans von Euler-Chelpin to investigate further. In 1929, they won the Nobel Prize for their research and increased understanding of the fermentation process; this included the chemical structure and properties of NAD+.
In 1930, Otto Warburg, himself a Nobel laureate, discovered that NAD+ played a key role in facilitating a myriad of biochemical reactions in the body. In particular, Warburg found that NAD+ was important in the transfer of electrons from one molecule to another. This transfer is the basis for the energy required for all biochemical reactions happening in the body.
In the 1940s and 50s, Arthur Kornberg’s research on NAD+ led to him discovering the mechanisms of DNA replication and RNA transcription.
In 1958, researchers Jack Preiss and Philip Handler discovered that there were three biochemical steps through which nicotinic acid is converted into NAD+. These three steps became known as the Preiss-Handler Pathway.
In 1976, Martin Rechsteiner found evidence that NAD+ had another key function beyond its already known role of electron transfer.
In 2000, Leonard Guarente published research that identified the activity of the sirtuin SIR2 as being dependent on the presence of NAD+. This reliance on NAD+ also likely explains how SIR2 links diet to physiology and the mechanism by which calorie restriction increases lifespan in multiple species.
What does NAD+ do?
NAD+ is important for the mitochondria, the powerhouses of our cells, to produce the energy our cells need. It functions as a coenzyme in the mitochondria, allowing the creation of chemical energy that our bodies can then use.
Metabolic processes such as glycolysis, the citric acid cycle (TCA/Krebs cycle), and the electron transport chain inside the mitochondria all rely on NAD+ in order to function.
In its function as a ligand, NAD+ binds to enzymes and transfers electrons between molecules.This means that NAD+ is found in two forms in the cell; NAD+ is an oxidizing agent that takes electrons from other molecules in order to become its reduced form, NADH. NADH can then become a reducing agent that donates the electrons it carries.
As electrons are the atomic basis of cellular energy, transferring them between molecules means that NAD+ works almost like recharging a battery. A battery goes flat because its electrons are used up to provide energy. The electrons cannot return to their charged state without a jolt, and it is the same in our cells. NAD+ gives the molecules the jolt they need to become active again, and in this way, NAD+ is able to increase or decrease enzyme activity, gene expression, and cell signalling.
How is NAD+ created?
Due to its critical importance in the body, most organisms can produce NAD+ in a few alternative ways. Humans like us have three major pathways to create NAD+: the de novo pathway, the Preiss–Handler pathway, and the salvage pathway.
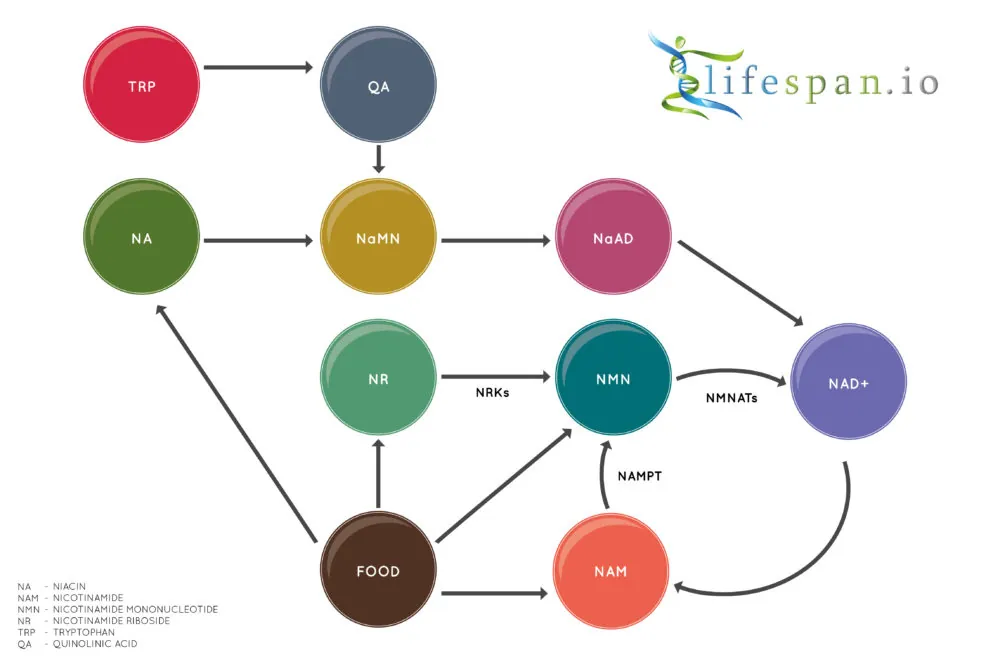
In biochemistry, de novo means that a molecule is created directly from another molecule: in the case of NAD+, the niacin molecule is built from scratch using the essential amino acid L-tryptophan (TRP). The de novo is the only non-vitamin B3 pathway for the creation of NAD+.
The Preiss–Handler pathway is one of the vitamin B3 pathways and starts with either nicotinic acid (NA) or niacinamide (NAR). The NA or NAR present in the food we consume is then converted via a series of enzymatic reactions into NAD+. As the above diagram shows, this pathway and the de novo pathway both join at the point where they become NaNM and continue along the same path to become NAD+. Niacin is perhaps the best known name for nicotinic acid, and as a 2020 human trial showed, niacin increases NAD+ significantly in muscle tissue.
Finally, the salvage pathway converts nicotinamide (NAM), also known as niacinamide, into NAD+. This pathway has nicotinamide mononucleotide (NMN) as an intermediate, and nicotinamide riboside (NR) also uses the same salvage pathway. This pathway is called the salvage pathway because instead of using the usual precursor molecules, such as TRP or Vitamin B3, to make NAD+, this pathway recycles (salvages) NAM, which is often generated from NAD+, back into NAD+ again.
Of the NAD+ pathways, perhaps the most recent focus has been on the salvage pathway and the NAD+ precursor molecules NMN and NR. There are now many supplement companies marketing NAD+ precursors as NAD+ boosters.
NAD+ helps repair DNA damage
NAD+ is a very useful coenzyme and is utilized by many proteins in the human body, particularly the sirtuins, which repair DNA damage and are associated with healthy longevity in multiple species. In this way, NAD+ helps to combat genomic instability, one of the proposed reasons we age.
Most cells contain the necessary repair systems to fix DNA damage. However, repairing this damage consumes NAD+, and if DNA damage becomes excessive, it can deplete the pool of NAD+ available and impact the other functions that require it.
An important protein involved in DNA repair is Poly (ADP-ribose) polymerase (PARP), which relies on the presence of NAD+ to work. The level of available NAD+ declines as we age, and DNA damage increases, which, in turn, elevates PARP, which then consumes even more NAD+ to repair it. Effectively, as we age, we are caught in a downward spiral of increasing NAD+ consumption from an ever-shrinking pool of available NAD+.
Experiments conducted in mice have demonstrated that treatment with the NAD precursor NMN can mitigate and resist age-related DNA damage as well as the damage resulting from exposure to radiation [1]. Of course, this data is taken from animal studies and is subject to the usual caveat that results may not be the same in humans; ongoing research has yet to determine if these results will translate.
NAD+ and the Sirtuins
The sirtuins, often referred to as the longevity genes, play a key role in maintaining healthy cellular function. The sirtuins are a family of enzymes that take part in cellular stress responses, facilitating cellular damage repair, and they are also involved in insulin production and secretion. Sirtuin activation is dependent on the availability of NAD+ in order to function, and they play an important role in aging.
David Sinclair, a Harvard geneticist and probably one of the most well known researchers on NAD+ biology, believes that the age-related loss of NAD+ along with the corresponding fall of sirtuin activity and its protective effects are a primary reason why we tend to develop diseases when we are old and why we do not when we are young. David Sinclair is a big proponent of NAD+ repletion and other methods of keeping our NAD+ levels up as we age to encourage healthy longevity.
David Sinclair and his team previously demonstrated that the NAD+ precursor NMN can extend the lifespan of mice in a previous study [2] and that it also reverses loss of mitochondrial function with age [3]. NMN also appears to address retinal degeneration via interaction with the mitochondrial sirtuins SIRT3 and SIRT5, at least in mice engineered to lack nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme in the production of NAD+ [4].
NAD+ and insulin
Mouse studies also suggest that NMN improves the production and activity of insulin. Treatment with NMN appeared to improve hepatic insulin resistance induced by a high-fat diet by restoring NAD+ synthesis, increasing the activity of NAD+ dependent SIRT1 and reducing the gene expressions linked with oxidative stress, inflammation, and circadian rhythm disruption [5].
Other studies suggest that long-term NMN consumption reduces age-related inflammation in adipose (fat) tissue and improves whole-body insulin sensitivity in normally aging mice [6]. NAD+ synthesis is impaired in obese and aged mice, so this study suggests that adipose tissue NAD+ could be a suitable target for insulin resistance, key risk factors for type 2 diabetes, and cardiovascular disease.
Once again, it should be noted that the above studies are in mice, and we must be mindful of this when considering whether the effects translate to humans.
The future of NAD+ research
The results from animal studies using various approaches to NAD+ repletion have significant potential in the context of aging and healthy longevity, but we are still in the early days. While there are a myriad of animal studies on the effects of NR and NMN on aging, there isn’t yet much human data supporting their use.
There was a human trial in Japan a few years ago using NMN, but this simply established that long-term consumption of NMN appeared to be well tolerated. There are more studies and human trials being conducted at the moment, and hopefully in the near future, there will be much more data upon which a conclusion can be formed about its use in the context of human aging and healthy longevity.
Disclaimer
This article is only a very brief summary, is not intended as an exhaustive guide, and is based on the interpretation of research data, which is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you. We do not endorse supplement use nor any product or supplement vendor, and all discussion here is for scientific interest.
Literature
[1] Li, J., Bonkowski, M. S., Moniot, S., Zhang, D., Hubbard, B. P., Ling, A. J., … & Sinclair, D. A. (2017). A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science, 355(6331), 1312-1317.
[2] North, B. J., Rosenberg, M. A., Jeganathan, K. B., Hafner, A. V., Michan, S., Dai, J., … & van Deursen, J. M. (2014). SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. The EMBO journal, e201386907.
[3] Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., … & Mercken, E. M. (2013). Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624-1638.
[4] Lin, J. B., Kubota, S., Ban, N., Yoshida, M., Santeford, A., Sene, A., … & Yoshino, J. (2016). NAMPT-mediated NAD+ biosynthesis is essential for vision in mice. Cell reports, 17(1), 69-85.
[5] Yoshino, J., Mills, K. F., Yoon, M. J., & Imai, S. I. (2011). Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet-and age-induced diabetes in mice. Cell metabolism, 14(4), 528-536.
[6] Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., … & Yoshino, J. (2016). Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell metabolism, 24(6), 795-806.