Niacin: Benefits, Side Effects, and Research
Niacin is a widely used dietary supplement with a long research history and several tricks up its sleeve. Recent human trials have shed new light on its possible role in addressing mitochondrial dysfunction and aging.
What is niacin?
Niacin (Vitamin B3) is a water-soluble vitamin discovered in 1937 by biochemist Conrad Elvehjem. It was initially used to treat pellagra, a disease caused by vitamin B3 deficiency that causes skin lesions, diarrhea, dementia, and even death. Niacin is the third of eight presently known B vitamins, which are also water-soluble and operate synergistically in energy metabolism and other processes.
Niacin was originally called nicotinic acid because it can be created by the oxidation of nicotine with nitric acid. However, people knew nicotine as the addictive chemical in tobacco, so the name niacin (NIcotinic ACid vitamIN) was used instead. However, niacin naturally appears in two forms, nicotinic acid and nicotinamide, each with different pharmaceutical properties.
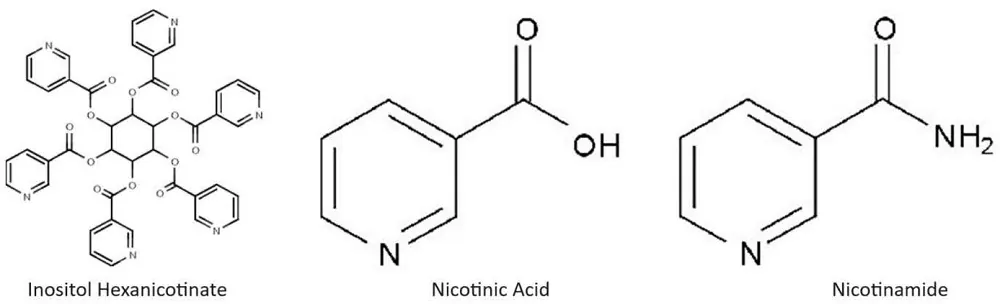
Additionally, nicotinic acid is sold in several slow or extended-release formulations to prevent an uncomfortable side effect known as “flushing.” Inositol hexanicotinate is one of these formulations sold over the counter—it is six nicotinic acid molecules attached to a single inositol molecule via ester bonds. When Inositol hexanicotinate (IH) enters the bloodstream and liver, esterases, enzymes that release nicotinic acid from inositol, are present.
Theoretically, these esterases would slowly liberate nicotinic acid and thus prevent flushing. However, studies suggest that only 70% of IH is absorbed in the gut, and most of its molecules remain intact when excreted. Both inositol hexanicotinate and nicotinamide are marketed as “no-flush” varieties of niacin [1]. However, their pharmacological effects are very different. Physicians prescribe most slow and extended-release nicotinic acid formulations. Doctors prescribe nicotinic acid to control cholesterol and associated lipid levels. These formulations remain in the body longer and can damage the liver [1].
Niacin in food
Foods rich in niacin include chicken, tuna, turkey, peanuts, coffee, kidney beans, pork, and bacon. Meats are generally the highest in niacin content by a large margin. However, this may not be practical for dietary reasons, as some people cannot eat or choose not to eat meat.
Fortunately, niacin supplements are available. The slow-release versions are sometimes called ‘delayed action’ or ‘persistent release’. Slow-release nicotinic acid is not recommended for regular supplementation, as it carries the risk of liver damage [2]. Slow-release nicotinic acid should only be consumed when directed by a qualified physician, and only for the stated duration.
The recommended daily amount of niacin for adult males is 16 milligrams (mg), and for adult women who aren’t pregnant, 14 mg, according to the National Academy of Sciences [3]. However, the liberal use of enriched flours in processed foods substantially increased the average niacin intake. Therefore, niacin deficiency, according to traditional criteria, is uncommon.
What’s the difference between regular niacin and “no-flush” niacin?
“No-flush” niacin can be inositol hexaniacinate (a different form of vitamin B3) and is not the same thing. Inositol hexanicotinate supports energy metabolism and the nervous system. Alternately, it can be nicotinamide, but no studies have shown that either has any effect on cholesterol levels, and they do not work in the same way as the nicotinic acid form of niacin. However, other extended-release forms of niacin do lower cholesterol. These varieties are generally not sold over the counter, and they carry a risk of liver damage and, paradoxically, vascular damage [1, 4].
What does niacin do?
Niacin is essential for normal nervous system function and maintaining healthy skin and mucous membranes. It helps the body convert food (carbohydrates) into fuel (glucose), which it uses to produce energy. This means that fatigue is a common sign of niacin deficiency. Niacin can also help reduce blood pressure.
As a precursor of nicotinamide adenine dinucleotide (NAD+), niacin can increase levels of NAD+ in cells. NAD+ is involved in the repair of DNA [5-6], and recently, the mechanism of how NAD+ repairs DNA was discovered [7].
In metabolism, NAD+ is a coenzyme involved in redox reactions, helping to move electrons from one reaction to another. NAD+ is an oxidizing agent; it accepts electrons from other molecules and becomes reduced. This reaction forms NADH, which is then used as a reducing agent to donate electrons. These electron transfer reactions are the primary function of NAD+, but NAD+ is also involved in other cellular processes. For example, it is associated with the sirtuins, which are proteins linked to mammalian longevity.
Read More
Niacin and cholesterol
Niacin increases high-density lipoprotein (HDL) cholesterol and reduces low-density lipoprotein (LDL) cholesterol [8-10], and both of these actions are generally beneficial. It is commonly used to control blood pressure and cholesterol levels in patients at risk of heart disease, dyslipidemia, hypercholesterolemia, or hyperlipidemia.
It blocks the production of very low-density lipoprotein (VLDL) in the liver and, consequently, its byproduct, LDL [11]. VLDL transports both triglycerides and cholesterol. Once in circulation, VLDL is broken down, releasing triglycerides for energy use by cells or storage in fat tissue. Once triglycerides are released, their composition changes into intermediate-density lipoprotein (IDL). Later, when the amount of cholesterol increases, IDL becomes LDL.
Niacin can raise HDL by as much as 30-35 percent. This effect is caused by reduced cholesterol transfer from HDL to VLDL and delayed clearance of HDL [12]. The drug also lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, and lipoprotein.
The levels of niacin required to significantly lower LDL and raise HDL deemed necessary by a physician are orders of magnitude higher than the levels commonly consumed by the general population. The median intake of niacin in the United States is 37 mg/day. Physician-prescribed dosages for LDL and HDL adjustment are 40-67 times higher (1500-2500 mg/day).
A study published in February 2024 by Mark S. Borja and colleagues suggests that niacin’s effects on HDL are both quantitative and qualitative. Borja explored the effects of niacin and omega-3 fatty acids on a specific aspect of HDL function in people with metabolic syndrome (MetS). This aspect, known as HDL-apolipoprotein A-I exchange (HAE), is crucial for HDL’s role in removing cholesterol from cells, a process called reverse cholesterol transport. While HAE has been used to study HDL function in observational research, this study aimed to see if treatments that change HDL’s composition, like niacin and omega-3 fatty acids, could affect HAE.
Fifty-six individuals with MetS participated in a double-blind trial. For 16 weeks, they received either a placebo, extended-release niacin (2g/day), prescription omega-3 ethyl esters (4g/day), or a combination of both. The researchers measured HAE at the start and end of the study period.
Results showed that niacin and omega-3 fatty acids alone significantly improved HAE by 15.1% and 11.1%, respectively, compared to placebo. However, the increase was 10.0% when combined, indicating that each substance might work better alone than together for this measure. Furthermore, the study found that niacin and omega-3 fatty acids could increase the efficiency of HDL without necessarily increasing HDL cholesterol (HDL-C) and apoA-I levels. These results suggest that the HAE assay, a test used in the study, can reveal improvements in HDL’s functionality that aren’t detected by traditional markers like HDL-C.
This research highlights that treatments with niacin and omega-3 fatty acids can enhance the functional properties of HDL in people with metabolic syndrome, offering potential benefits beyond those indicated by standard blood tests [13].
Niacin and chronic obstructive pulmonary disease
In a 2024 study by Li and colleagues examining the link between dietary niacin intake and chronic obstructive pulmonary disease (COPD) among American adults, researchers used data from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2018. They gathered information on niacin intake through dietary interviews and identified COPD cases based on lung function tests, medical history, and medication usage.
Out of 7,055 participants, 243 were diagnosed with COPD. The analysis revealed that individuals with COPD consumed less Niacin on average (21.39 mg/day) than those without COPD (25.29 mg/day). After adjusting for various factors, the study found that people in the highest quartile of niacin intake were significantly less likely to have COPD than those in the lowest quartile, suggesting an inverse relationship between niacin intake and COPD prevalence.
This research implies that consuming higher amounts of dietary Niacin could potentially lower the odds of developing COPD, highlighting the need for further studies to explore dietary niacin supplementation as a preventive strategy against COPD [14].
Niacin and heart disease
The CLAS study, a two-part, randomized, placebo-controlled, angiographic trial, combined colestipol-niacin therapy in 162 subjects [10]. Two-year results (CLAS-I) showed a decreased progression of atherosclerosis and an increased regression. Researchers treated a subgroup of 103 subjects for four years (CLAS-II). They monitored blood lipids, lipoprotein-cholesterol, and apolipoprotein during the trial. After four years, many subjects showed non-progression (52% vs. 15% placebo-treated) of coronary artery lesions. Also, some saw regression (18% vs. 6% placebo-treated) of coronary artery lesions.
Significantly fewer drug-treated subjects developed new lesions in native coronary arteries (14% vs. 40% placebo-treated) and bypass grafts (16% vs. 38% placebo-treated). These results confirm the CLAS-I findings and indicate that regression can continue for at least four years.
Targeting patients with coronary disease and low HDL cholesterol, the HATS study looked at niacin plus simvastatin, antioxidant-vitamin therapy, a combination of these therapies, and a placebo [15]. The antioxidant therapy included vitamin E, 1000 mg of vitamin C, 25 mg of natural beta-carotene, and 100 μg of selenium. Simvastatin plus niacin provided multiple measurable benefits against coronary artery blockages compared to antioxidant-vitamin therapy and placebo.
These studies didn’t tackle whether niacin intake prevented heart attacks and strokes or reduced deaths from heart conditions. While the intuitive answer might be yes, the role of niacin in lowering cardiovascular disease (CVD) risk has been brought into doubt [16, 17].
Recent research indicates that despite niacin’s ability to lower LDL cholesterol and improve HDL cholesterol and triglyceride levels, its combination with high-potency statins doesn’t reduce CVD risk [18, 19]. Furthermore, a meta-analysis found that niacin may increase the risk of death [20].
In a study led by Elvira D’Andrea and colleagues in April 2019, researchers examined how effective niacin is for patients with heart diseases. They reviewed 119 clinical trials involving more than 35,000 participants to see if niacin could prevent heart-related diseases. Of these, 17 trials specifically examined how niacin affects heart disease outcomes. The findings could have been more consistent. On one hand, niacin didn’t prevent heart diseases across the board, but when they looked closer, particularly at patients who were only taking niacin without any other treatments, they found some benefits in reducing heart disease events.
The key takeaway from this research is that niacin might still have a role, especially for patients who can’t tolerate statins (a common heart disease medication), but only for controlling lipid (fat) levels in the blood [21].
In February 2024, Nature Magazine published a study by Marc Ferrell focusing on the persistent risk of cardiovascular disease (CVD) despite rigorous preventive measures and adherence to established treatment guidelines. Ferrell’s team focused on niacin, examining fasting plasma samples from a sizable cohort of stable cardiac patients (1,162 participants, including 422 females). Their efforts uncovered a link between niacin metabolism and the occurrence of major adverse cardiovascular events (MACE).
This study identified that elevated serum levels of niacin’s breakdown products, (2PY) and (4PY), indicated a higher risk of experiencing MACE within three years. The research group validated this cohort across two distinct cohorts, one from the US (2,331 participants, 774 females) and another European group (832 participants, 249 females), with adjusted hazard ratios pointing to significant risk increases linked to 2PY and 4PY levels.
Further investigation led the research group to discover a genetic variant, rs10496731, influencing 2PY and 4PY levels. This variant also showed a notable correlation with the levels of soluble vascular adhesion molecule 1 (sVCAM-1), a key player in vascular inflammation and atherosclerosis. A subsequent meta-analysis involving a vast pool of participants (106,000 individuals, including 53,075 females) solidified the connection between rs10496731 and sVCAM-1.
Moreover, the study observed a direct relationship between sVCAM-1 levels and the niacin metabolites 2PY and 4PY in a validation cohort (974 participants, including 333 females), further underpinning the biological significance of these findings. Mouse experiments showed that PY could trigger the expression of VCAM-1 and promote leukocyte adherence to vascular endothelium, suggesting a potential inflammatory mechanism linking 4PY to increased MACE risk.
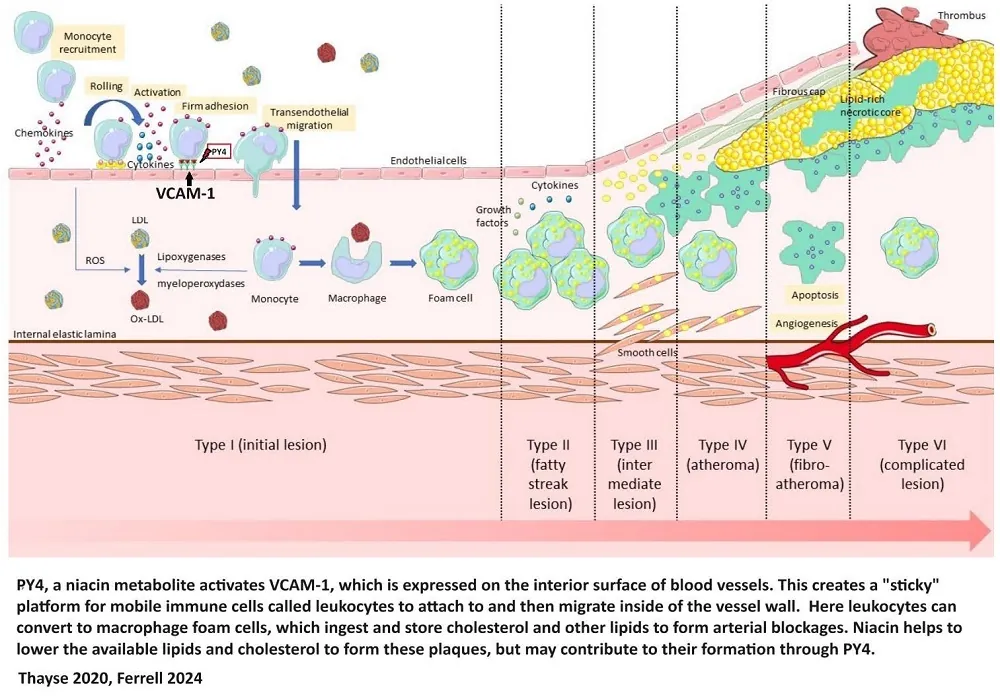
Ferrell’s research speaks to the complex role that excess niacin and its metabolites, 2PY and 4PY, play in cardiovascular health. These findings not only highlight the subtle relationship between niacin metabolism and residual CVD risk but also propose an inflammation-dependent mechanism that could explain the clinical association between 4PY levels and the occurrence of major adverse cardiovascular events [4]. These findings were in cardiac patients taking prescription doses of niacin.
The failure of the expected decrease in cardiovascular disease outcomes to materialize despite substantial improvements in patient lipid profiles has been dubbed the niacin paradox. Several phenomena may account for this apparent discrepancy between improved lipid profiles and negligible changes in mortality, including the fact that LDL and HDL are only two factors involved in heart disease. Other factors, such as inflammation, may be worsened by niacin [4].
Another factor is the functionality of HDL particles, including their role in reverse cholesterol transport and inflammation modulation. High-density lipoproteins (HDL) are critical to cholesterol management. However, the quality and functionality of HDL are equally important as its quantity. Quality involves the HDL’s composition, protein, and lipid content and whether it has undergone oxidation or glycation. Functionality refers to HDL’s ability to protect against oxidation, reduce inflammation, and remove cholesterol from cells.
The quantity and quality of HDL can vary throughout life due to diet, exercise, smoking, age, and gender. For instance, puberty, aging, and menopause can all affect HDL-C levels. Diseases, acute infections, and chronic inflammation can decrease HDL-C, whereas regular aerobic exercise and a healthy diet can increase it. Generally, having a higher HDL-C level is considered beneficial, but a high quantity of HDL doesn’t guarantee its quality or effectiveness [22].
Niacin may also produce a host of off-target effects. For example, nicotinic acid attaches to the GPR109A skin receptor, which is what enables it to cause flushing. This receptor is found in the skin and on the surface of other cells, including fat cells, nerve cells, gut epithelial, and immune cells. In each case, the signaling networks activated are different. More research is needed to understand the downstream effects of GPR109A signaling fully. Despite niacin’s long history, it is the subject of much ongoing research [23-26].
Niacin and diabetes
A 2016 study suggested that niacin increases blood glucose levels, prompting the idea that it may contribute to new-onset diabetes. A meta-analysis of 11 randomized trials was conducted to confirm whether or not such a link exists [27].
These trials were found by searching the Cochrane database and EMBASE between 1975 and 2014. Inclusion criteria demanded randomized controlled trials on niacin and its cardiovascular effects on 50 or more non-diabetic participants. The research group conducted this study as a two-armed study with 26,340 participants; of these, 13,121 were assigned to the niacin therapy group, and 13,219 were assigned to the control group.
Of the 26,340 participants analyzed, 725 in the niacin group and 646 in the control group had developed new-onset diabetes. Compared to placebo, niacin was shown to be associated with a moderately increased risk of developing diabetes. However, the cardiovascular benefits of niacin therapy may outweigh the risk of developing diabetes.
Niacin increased NAD+ in human trials
In 2020, a human trial showed that niacin increases NAD+ significantly [28]. Researchers gave participants an escalating dose of niacin, starting at 250 mg daily and rising to 750-1000 mg daily over four months. Finally, at the 10-month follow-up treatment, the participants formed two groups: people with mitochondrial myopathy and healthy age-matched people. There were two healthy people for each patient with mitochondrial myopathy. All participants in the trial were given the same escalating niacin regimen.
The researchers reported that niacin increased muscle NAD+ levels by 1.3-fold by the 4-month mark. NAD+ further increased to 2.3-fold after ten months in the mitochondrial myopathy group. The healthy control group saw no such increase, suggesting that NAD+ levels are regulated in skeletal muscle tissue and only increase when levels are below average, as in mitochondrial myopathy. The tendency to only increase when levels are below average may also be the case during aging, where efficient mitochondrial function is reduced.
Whole-blood NAD+ also increased by 7.1-fold in the study group and 5.7 in the control group after four months compared to the participants’ baseline. By the 10-month mark, it had increased 8.2-fold compared to baseline. The results confirm that niacin reaches the bloodstream in significant amounts without removal by the liver.
Niacin appears to improve body composition
That trial’s researchers also reported that niacin improved body composition. Participants saw a decrease in whole-body fat percentage in controls and increased muscle mass in both the control and study groups. After ten months, participants saw increased muscle strength. They noted that hepatic fat was reduced by half and visceral fat by a quarter; both of these fat deposits are associated with an increased risk of metabolic syndrome.
The researchers also considered the risk of niacin increasing blood glucose levels. This study showed that niacin increased fasting glucose levels in both groups following four months of supplementation. However, glycosylated hemoglobin, which reflects long-term glucose levels, was unaffected [28].
Niacin and the nervous system
In 2023, Ozaydin’s research group published a study on the impact of niacin on inflammation, oxidative stress, and the process of cell death in the context of mild traumatic brain injury (TBI). The study involved 25 male rats, divided into three groups: a control group, a TBI + placebo group, and a TBI + niacin group (receiving 500 mg/kg of niacin). Ozaydin’s group induced TBI under anesthesia, and behavioral tests were conducted before and 24 hours after the injury to assess the impact. The researchers measured indicators of oxidative stress and tissue cytokine levels and examined the brain tissue for signs of damage.
The findings showed niacin treatment noticeably reduced the effects of TBI. Niacin was also found to elevate levels of the anti-inflammatory cytokine IL-10, which the injury had reduced. Moreover, niacin treatment effectively reduced histological damage to different parts of the rats’ brains [29].
In a United States study led by Leiyong Zhao, published in 2023, researchers examined how the amount of niacin a person eats is related to depression. They discovered a unique U-shaped connection, indicating that both too little and too much niacin intake can have an impact on depression, with the turning point being at 26.6 milligrams a day.
This pattern held across various groups, including men, Mexicans, United States citizens, White people, those under 40 years old, and individuals with a body mass index (BMI) less than 30. This finding suggests that while niacin is essential for mental health, there’s a sweet spot in how much someone should consume. Too little might not provide enough support for mental health, but too much could potentially worsen it [30].
In a study led by Walaa Wadie published in 2023, researchers explored how niacin could affect depression linked with inflammatory bowel disease (IBD) in rats. Depression often occurs alongside IBD, affecting the overall well-being and treatment response of patients.
The results showed that niacin helped improve the depressive behaviors brought on by colitis and lessened the inflammation in the colon, as observed visually and under a microscope. Notably, niacin appeared to help repair the blood-brain barrier (BBB), which is crucial in preventing harmful bodily substances from entering the brain.
Increased levels of specific proteins in the hippocampus, a vital brain area for emotion and memory, indicated this. Additionally, niacin reduces levels of pro-inflammatory markers and boosts substances related to reducing oxidative stress and inflammation in the brain. However, some of these beneficial effects were lessened when the rats were given a GPR109A blocker along with niacin.
This study highlights niacin’s potential to address the physical symptoms of IBD and counteract related depressive-like symptoms through mechanisms that involve the GPR109A receptor, suggesting a new area of treatment for the neurological side effects of IBD [31].
Niacin as a potential treatment for liver cancer
In a study by Nemany published in 2023, researchers explored a new way to fight liver cancer cells using natural ingredients. They experimented with tiny bubbles (liposomes) coated in chitosan, a natural substance that makes the bubbles more stable and better at carrying niacin into the cancer cells. They also added curcumin, a substance from turmeric, into these bubbles to help fight the cancer. Folic acid was used to target the cancer cells more precisely.
Their tests showed that the liposomes could significantly slow down the growth of liver cancer cells after 48 hours. They looked at how much the cancer cells grew compared to normal and found a considerable decrease when the cells were treated with these bubbles filled with niacin and curcumin.
The study suggests that using natural compounds like niacin and curcumin packaged in liposomes could offer a new way to treat liver cancer [32].
Niacin and erectile dysfunction
In a study published in January 2024 by Lin Wei-Long and colleagues in the Asian Journal of Andrology, researchers aimed to fill the gap in understanding the connection between dietary niacin intake and erectile dysfunction (ED). Utilizing data from the 2001–2004 National Health and Nutrition Examination Survey (NHANES) in the USA, they analyzed the dietary habits of 3,184 adults. Among these participants, 863 were reported to have ED.
The study applied advanced statistical methods to assess the impact of niacin intake on ED risk. Their findings indicated a significant link between higher niacin intake and a lower risk of ED. Specifically, individuals within the top third of niacin consumption were found to have a 44% lower risk of developing ED compared to those in the bottom third. This relationship remained consistent across different subgroups and was confirmed by dose–response curves, suggesting that as dietary niacin intake increased, the risk of ED decreased [33].
Niacin Side effects
A typical side effect of high-dose niacin is the “niacin flush”, a reaction that can potentially cause a burning, tingling, and itching sensation on the skin. This flushing is harmless and typically subsides within 30 minutes to an hour. This flush is generally most intense after the first dose and diminishes with continued use of niacin as the body grows used to it. Its severity may also be reduced by starting at a low dose (50-100 mg), taking an aspirin or white willow extract beforehand, and drinking water.
Slow-release/sustained-release niacin carries the risk of liver damage [1]. Anyone experiencing serious side effects should cease taking niacin immediately and consult a medical professional.
There is also some concern that niacin can deplete methyl groups [34] and raise homocysteine, an amino acid. Vitamins B12, B6, and folate break down homocysteine to create other molecules, but high homocysteine levels are a risk factor for heart attacks [35]. It may be possible to reduce homocysteine levels by restoring methyl groups using supplements such as trimethylglycine (TMG).
Disclaimer
This article is only a brief summary, not intended as an exhaustive guide, and is based on the interpretation of research data, which is speculative. It is not a substitute for consulting your physician about which supplements may or may not suit you. We do not endorse supplement use or any product or supplement vendor; all discussion here is for scientific interest.
Literature
[1] Mackay, D.; Hathcock, J.; Guarneri, E. Niacin: Chemical Forms, Bioavailability, and Health Effects. Nutr Rev 2012, 70, 357–366.
[2] Rader, J. I., Calvert, R. J., & Hathcock, J. N. (1992). Hepatic toxicity of unmodified and time-release preparations of niacin. The American journal of medicine, 92(1), 77-81.
[3] Dietary Reference Intakes: The Essential Guide to Nutrient Requirements | The National Academies Press
[4] Ferrell, M.; Wang, Z.; Anderson, J.T.; Li, XS; Witkowski, M.; DiDonato, JA; Hilser, JR; Hartiala, J.A.; Haghikia, A.; Cajka, T.; et al. A Terminal Metabolite of Niacin Promotes Vascular Inflammation and Contributes to Cardiovascular Disease Risk. Nature Medicine 2024 30:2 2024, 30, 424–434.
[5] Kennedy, D. O. (2016). B vitamins and the brain: Mechanisms, dose and efficacy—A review. Nutrients, 8(2), 68.
[6] Kirkland, J. B. (2012). Niacin requirements for genomic stability. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 733(1), 14-20.
[7] Li, J., Bonkowski, M. S., Moniot, S., Zhang, D., Hubbard, B. P., Ling, A. J., … & Aravind, L. (2017). A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science, 355(6331), 1312-1317.
[8] Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–98.
[9] Kamanna VS, Kashyap ML. Mechanism of action of niacin on lipoprotein metabolism. Curr Atheroscler Rep. 2000;2:36–46.
[10] Cashin-Hemphill L, Mack WJ, Pogoda JM, et al. Beneficial effects of colestipol-niacin on coronary atherosclerosis. A 4-year follow-up. JAMA. 1990;264:3013–7.
[11] Grundy, S. M., Mok, H. Y. L., Zech, L., & Berman, M. (1981). Influence of nicotinic acid on metabolism of cholesterol and triglycerides in man. Journal of lipid research, 22(1), 24-36.
[12] Illingworth, D. R., Stein, E. A., Mitchel, Y. B., Dujovne, C. A., Frost, P. H., Knopp, R. H., … & Greguski, R. A. (1994). Comparative effects of lovastatin and niacin in primary hypercholesterolemia: a prospective trial. Archives of internal medicine, 154(14), 1586-1595.
[13] Borja, M.S.; Hammerson, B.; Tang, C.; Juarez-Serrano, L.; Savinova, O. V.; Harris, W.S.; Oda, M.N.; Shearer, G.C. Effects of Niacin and Omega-3 Fatty Acids on HDL-Apolipoprotein A-I Exchange in Subjects with Metabolic Syndrome. PLoS One 2024, 19, e0296052.
[14] Li, WW; Ren, K.L.; Yu, J.; Guo, H.S.; Liu, B.H.; Sun, Y. Association of Dietary Niacin Intake with the Prevalence and Incidence of Chronic Obstructive Pulmonary Disease. Scientific Reports 2024 14:1 2024, 14, 1–9.
[15] Brown, B. G., Zhao, X. Q., Chait, A., Fisher, L. D., Cheung, M. C., Morse, J. S., … & Frohlich, J. (2001). Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New England Journal of Medicine, 345(22), 1583-1592.
[16] Landmesser, U. The Difficult Search for a “partner” of Statins in Lipid-Targeted Prevention of Vascular Events: The Re-Emergence and Fall of Niacin. Eur Heart J 2013, 34, 1254–1257.
[17] Lloyd-Jones, DM Niacin and HDL Cholesterol–Time to Face Facts. N Engl J Med 2014, 371, 271–273.
[18] Landray, M, et al. Effects of Extended-Release Niacin with Laropiprant in High-Risk Patients. N Engl J Med 2014, 371, 203–212.
[19] Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. New England Journal of Medicine 2011, 365, 2255–2267.
[20] Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y. in; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J Am Coll Cardiol 2018, 71, 2570–2584.
[21] D’Andrea, E.; Hey, S.P.; Ramirez, C.L.; Kesselheim, A.S. Assessment of the Role of Niacin in Managing Cardiovascular Disease Outcomes: A Systematic Review and Meta-Analysis. JAMA Netw Open 2019, 2, e192224–e192224.
[22]Cho, K.H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. International Journal of Molecular Sciences 2022, Vol. 23, Page 3967 2022, 23, 3967.
[23] Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nature Communications 2015 6:1 2015, 6, 1–15, doi:10.1038/ncomms7734.
[24] Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-Protein-Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res 2009, 69, 2826–2832.
[25] Plaisance, E.P.; Lukasova, M.; Offermanns, S.; Zhang, Y.; Cao, G.; Judd, R.L. Niacin Stimulates Adiponectin Secretion through the GPR109A Receptor. Am J Physiol Endocrinol Metab 2009, 296, 549–558.
[26] GPR109A Is a G-Protein–Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon | Cancer Research | American Association for Cancer Research.
[27] Goldie, C., Taylor, A. J., Nguyen, P., McCoy, C., Zhao, X. Q., & Preiss, D. (2016). Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart, 102(3), 198-203.
[28] Pirinen, E., Auranen, M., Khan, N. A., Brilhante, V., Urho, N., Pessia, A., … & Haimilahti, K. (2020). Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metabolism.
[29] Ozaydin, D.; Bektasoglu, P.K.; Koyuncuoglu, T.; Ozkaya, S.C.; Koroglu, A.K.; Akakin, D.; Erzik, C.; Yuksel, M.; Yegen, B.C.; Gurer, B. Anti-Inflammatory, Antioxidant and Neuroprotective Effects of Niacin on Mild Traumatic Brain Injury in Rats. Turk Neurosurg 2023, 33, 1028–1037.
[30] Zhao, L.; Guo, S.; Yang, J.; Wang, Q.; Lu, X. Association between Niacin Intake and Depression: A Nationwide Cross-Sectional Study. J Affect Disord 2023, 340, 347–354, doi:10.1016/J.JAD.2023.08.053.
[31] Wadie, W.; Mohamed, S.S.; Abd El-Haleim, E.A.; Khayyal, M.T. Niacin Modulates Depressive-like Behavior in Experimental Colitis through GPR109A-Dependent Mechanisms. Life Sci 2023, 330, 122004, doi:10.1016/J.LFS.2023.122004.
[32] Hanafy, N.A.N.; Sheashaa, R.F.; Moussa, E.A.; Mahfouz, M.E. Potential of Curcumin and Niacin-Loaded Targeted Chitosan Coated Liposomes to Activate Autophagy in Hepatocellular Carcinoma Cells: An in Vitro Evaluation in HePG2 Cell Line. Int J Biol Macromol 2023, 245, 125572.
[33] Lin, W.-L.; Zheng, C.; Wang, H.-X.; Zhang, W.; Lin, M.-E. Relationship between Dietary Niacin Intake and Erectile Dysfunction: A Population-Based Study. Asian J Androl 2024,.
[34] Conze, D., Brenner, C., & Kruger, C. L. (2019). Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Scientific reports, 9(1), 1-13.
[35] Chrysant, S. G., & Chrysant, G. S. (2018). The current status of homocysteine as a risk factor for cardiovascular disease: a mini-review. Expert review of cardiovascular therapy, 16(8), 559–565.


