Antinuclear Antibody Shows Promise Against Cancer
- This approach bypasses one of the hardest problems in oncology.

Scientists have developed a conjugate of a drug and a nucleus-targeting antibody that can attack multiple types of cancer cells without targeting a particular antigen [1].
The anti-nuclear missile
Antinuclear antibodies (ANA) are usually associated with autoimmune diseases, such as lupus, where those antibodies attack cellular nuclei, binding to nucleic acids and other local molecules. ANAs’ ability to penetrate cellular membranes has drawn oncologists’ attention because ANAs can be conjugated with drugs and deliver them into tumor cells.
Some ANAs have an interesting Trojan horse-like strategy for sneaking into cells. They bind to extracellular nucleic acids and short chunks of DNA (nucleosomes) and piggyback on salvage pathways that transport those useful molecules back into cells [2]. In this new study, scientists from Yale university used this mechanism to attack cancer.
In fast-growing tumors, some cells do not get enough oxygen and nutrients and die off. This process, called tumor necrosis, is indicative of the tumor’s aggressiveness.
Those dead cells emit a lot of nucleosides, which are precursor molecules to nucleotides, DNA’s building blocks. Living cancer cells employ transporter proteins to bring those nucleosides back in where they can be reused.
Exploiting this effect, the researchers developed an antinuclear antibody-drug conjugate (ANADC) that targets those tumor-specific “nucleoside junkyards” and hitches a ride into tumor cells with those same transporter proteins. Upon uptake by the cell, ANADCs are cleaved by the protein cathepsin B, and the drug (the division-preventing agent monomethyl auristatin E, or MMAE) is released into the cytoplasm. The researchers describe their invention as “an anti-nuclear missile.”
Tumor growth suppressed
The researchers tested their invention on the U87 cell line, which is an established model of brain cancer. Treatment with ANADC caused a drastic decrease in the cells’ viability. Conversely, other approaches, such as an antibody-drug conjugate based on a non-antinuclear antibody, failed to achieve noticeable cytotoxicity.
In a mouse model of triple-negative breast cancer, the treatment completely suppressed tumor growth, while antibodies and non-antinuclear antibody-drug conjugates did not. The mice did not experience weight loss, which suggests a lack of off-target toxicity. ANADC was still effective, although not as drastically, in a model of colon cancer.
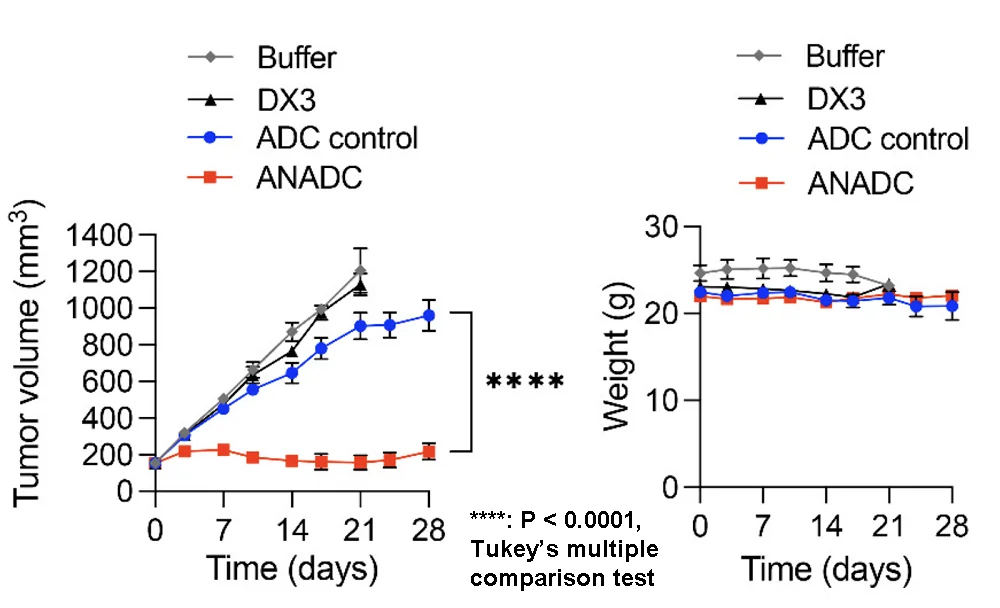
Crossing the blood-brain barrier
The toughest test, however, was brain tumors. Those hide behind the blood-brain barrier (BBB), which is impervious to most antibodies and antibody-drug conjugates. Nucleoside transporters, however, know how to traverse it.
The researchers confirmed that in a mouse U87 model of glioma, ANADC were indeed able to cross the BBB using the nucleoside transporter ENT2 and to target tumors. The treatment significantly increased the length of survival, although it did not cure the cancer completely. Importantly, advanced brain cancers are among the deadliest and fastest growing. No significant off-target deposition of ANADC was detected, suggesting high anti-cancer specificity.
According to the researchers, their invention has important advantages over current treatments, the most obvious one being that it does not target tumor cells via a specific antigen. Identifying the antigen that can be targeted in oncology is a complex and multifaceted process due to tumors’ genetic heterogeneity and other factors [3]. Pending further improvements, ANADC can potentially become an effective and fast off-the-shelf option for treating multiple cancer types.
The ANADC developed here targets the nucleic acid exhaust released by necrotic tumor cells and exploits mechanisms of nucleoside salvage by live cancer cells in the area as a DNA-seeking “antinuclear missile”. Antibody localization to extracellular nucleic acid waste helps mitigate concerns over target antigen depletion during therapy as tumor cell turnover and death yield a continuously renewing source of nucleic acids to draw ANADC to tumor microenvironments.
Literature
[1] Cao, F., Tang, C., Chen, X., Tu, Z., Jin, Y., Turk, O. M., … & Hansen, J. E. (2024). Cathepsin B Nuclear Flux in a DNA-Guided “Antinuclear Missile” Cancer Therapy. ACS Central Science.
[2] Weisbart, R. H., Chan, G., Jordaan, G., Noble, P. W., Liu, Y., Glazer, P. M., … & Hansen, J. E. (2015). DNA-dependent targeting of cell nuclei by a lupus autoantibody. Scientific Reports, 5(1), 1-6.
[3] Balibegloo, M., Keshavarz-Fathi, M., & Rezaei, N. (2021). Tumor Antigen Identification for Cancer Immunotherapy. Cancer Immunology: Bench to Bedside Immunotherapy of Cancers, 53-59.