Biomarkers of Aging for Facilitating Future Research
- Good biomarkers are how people can know that a treatment is working.

The Biomarkers of Aging Consortium, an initiative uniting about 200 geroscientists around the goal to inform and guide the use of biomarkers of aging, has put out its first paper [1].
The need for biomarkers
Since studies with lifespan as their endpoint can take a prohibitively long time, especially in humans, the importance of reliable biomarkers of aging is hard to overstate. Such biomarkers are essential for devising and validating anti-aging interventions and understanding the fundamental mechanisms of aging. While a lot of progress has been seen in this field, with established biomarkers such as methylation clocks being perfected, and multiple other types of biomarkers being introduced, there are still many obstacles to overcome.
The Biomarkers of Aging Consortium unites about 200 prominent geroscientists in an attempt to outline an approach to biomarkers of aging and standardize some of its important aspects. This was reported by Steve Horvath, “the father of methylation clocks”, at our recent conference in New York. The consortium has recently issued its first paper, which was published in Cell, and it has all the chances of becoming a seminal work on biomarkers of aging, guiding efforts in this field for years to come.
Standardizing definitions
This paper offers an overview of the history and the current state of the field, listing leading existing biomarkers of human aging, their scientific and commercial applications, and some of the ongoing clinical trials in which they are being used.
More importantly, it finally provides several crucial definitions, starting with the definition of aging itself. This paper defines aging as “the process of accumulation of consequences of life, such as molecular and cellular damage, that leads to functional decline, chronic diseases, and ultimately mortality”. This might be the first agreed-upon definition of aging, even if it has yet to be accepted by the longevity community as a whole.
An aging biomarker is defined in the paper as “a quantitative parameter of an organism that either alone or in a composite predicts biological age and ideally its changes in response to interventions.”
The definition of biological age is a bit longer but is worth quoting here in full: “Conceptually, an individual’s age defined by the level of age-dependent biological changes, such as molecular and cellular damage accumulation. In practical use, this is often summarized as a number (in units of time) matching the chronological age where the average person in a reference population shares the individual’s level of age-dependent biological changes.”
Age acceleration is defined as “the difference between biological age and chronological age (originally defined by Horvath and typically expressed in units of time)”. The authors add that they “propose adoption of the term age deviation (AgeDev) for this concept to distinguish it from an increased rate of aging and encompass changes in both directions.” Age deviation, indeed, seems to be a better term than “age acceleration” and will probably gain traction.
From those definitions naturally arises a concise definition of geroprotectors: “An agent or intervention that increases healthspan or lifespan and ameliorates [tested] biomarkers of aging”.
Classification and validation
The authors classify biomarkers of aging into types. The first one is molecular, based on ‘omics’, such as epigenomics, proteomics, or metabolomics, on individual molecules, such as IL-6 or IGF-1, or on composites of blood markers. The second type is physiological markers (VO2 max, gait speed, grip strength, etc.).
A third category is proposed as well: digital biomarkers, defined as coming from wearable devices or other tools that allow individuals to directly collect their own health data. However, many such biomarkers are at the same time either functional (such as heart rate variability), or molecular (such as data collected via continuous glucose monitoring). These biomarkers are becoming increasingly valuable, especially due to their intrinsically longitudinal nature.
In addition to their type, biomarkers can also be classified by their clinical applications, although no aging biomarkers of any category have yet been approved for clinical applications, at least in the US). In this context, biomarkers can, for instance, be predictive of a certain outcome, be it the onset of disease or the effect of an intervention. Prognostic biomarkers are similar to predictive ones, but they are applied to already diseased people to predict disease progression and outcome.
Response biomarkers are those that “indicate the biological reaction of an individual to an exposure or an intervention”, instead of just predicting outcomes such as mortality. Response biomarkers are a major stepping-stone on the way to surrogate endpoint biomarkers, which could truly power clinical studies of anti-aging interventions. For instance, reduction in blood pressure is a recognized surrogate endpoint for reduction in rates of stroke.
There are no FDA-recognized surrogate endpoints of aging yet, although epigenetic clocks, being used in many ongoing trials, probably come closest. More studies are required to determine whether those clocks or other biomarkers of aging are actually predictive of clinical outcomes, which is essential for them becoming surrogate endpoint biomarkers.
Finally, discovery biomarkers are those that can be effectively used to discover new targets and longevity interventions. Biomarkers based on large-scale omics data and powered by AI seem to be well-positioned to do so.
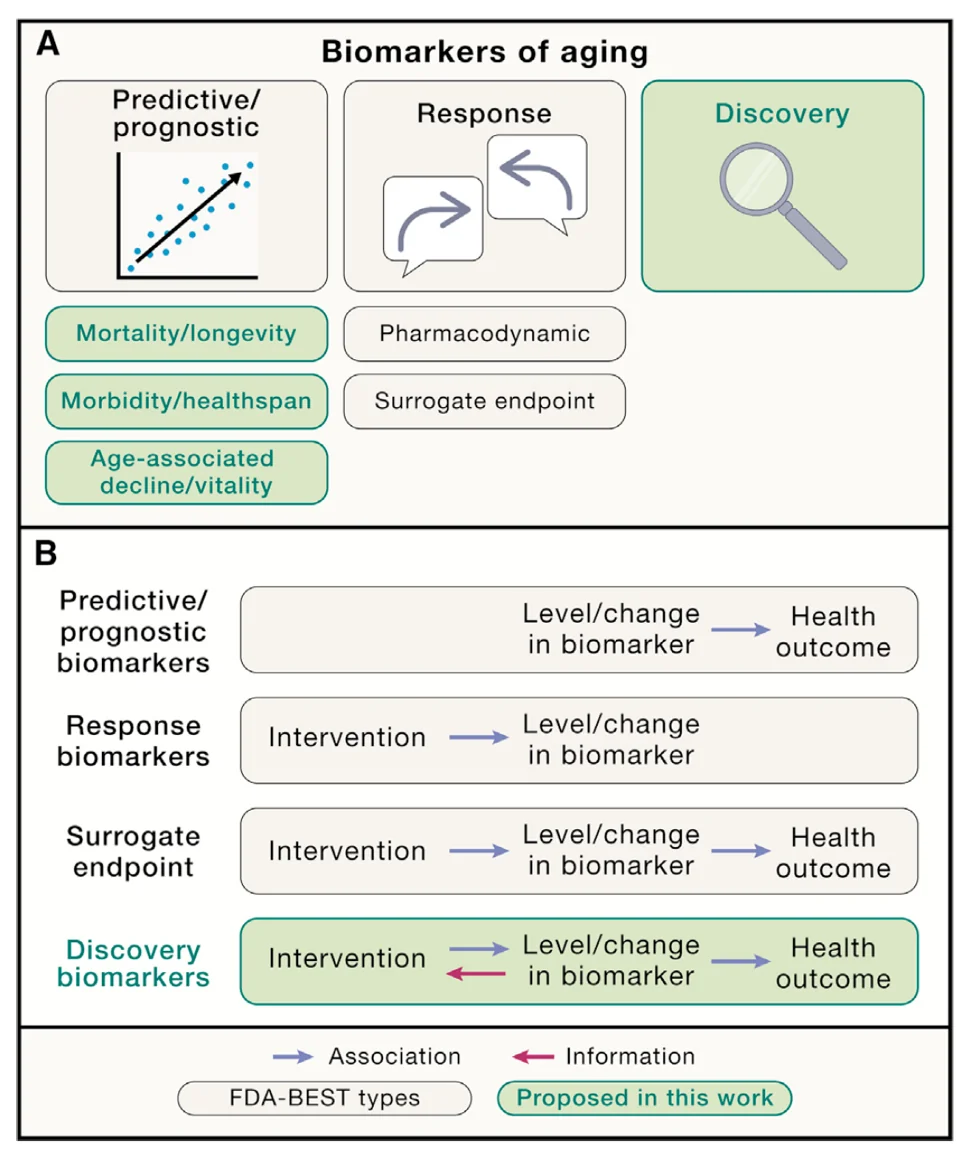
The authors also provide several important recommendations on how to rank and validate biomarkers using criteria such as minimal invasiveness; high reliability (i.e., low variability); relevance to aging; ability to predict functional aspects of aging, such as mortality, better than chronological age; and responsiveness to longevity interventions. These criteria are neither necessary nor sufficient but rather provide a framework for assessing to what extent candidate biomarkers might be feasible, valid, and useful.
It may be unrealistic to identify a single biomarker that captures all aspects of biological aging and satisfies all criteria. Each biomarker of aging has advantages and limitations, which may be evaluated using this framework.
In line with what one of this paper’s authors, Vadim Gladyshev of Harvard, said at our conference, the paper suggests that cross-species applicability is indicative of a good biomarker of aging. Biomarkers that work in animal models are indispensable for pre-clinical research, while cross-species compatibility allows to apply the same biomarkers in clinical research. Importantly, biomarkers should also function across different human populations.
More biomarkers in more cohorts
The authors outline a seemingly cyclical relationship between geroprotectors (supposedly life-prolonging interventions) and biomarkers of aging, which can help to validate both: “There exist many candidate biomarkers of aging and many proposed geroprotectors, and the accumulated evidence supporting either could be leveraged to validate the other.”
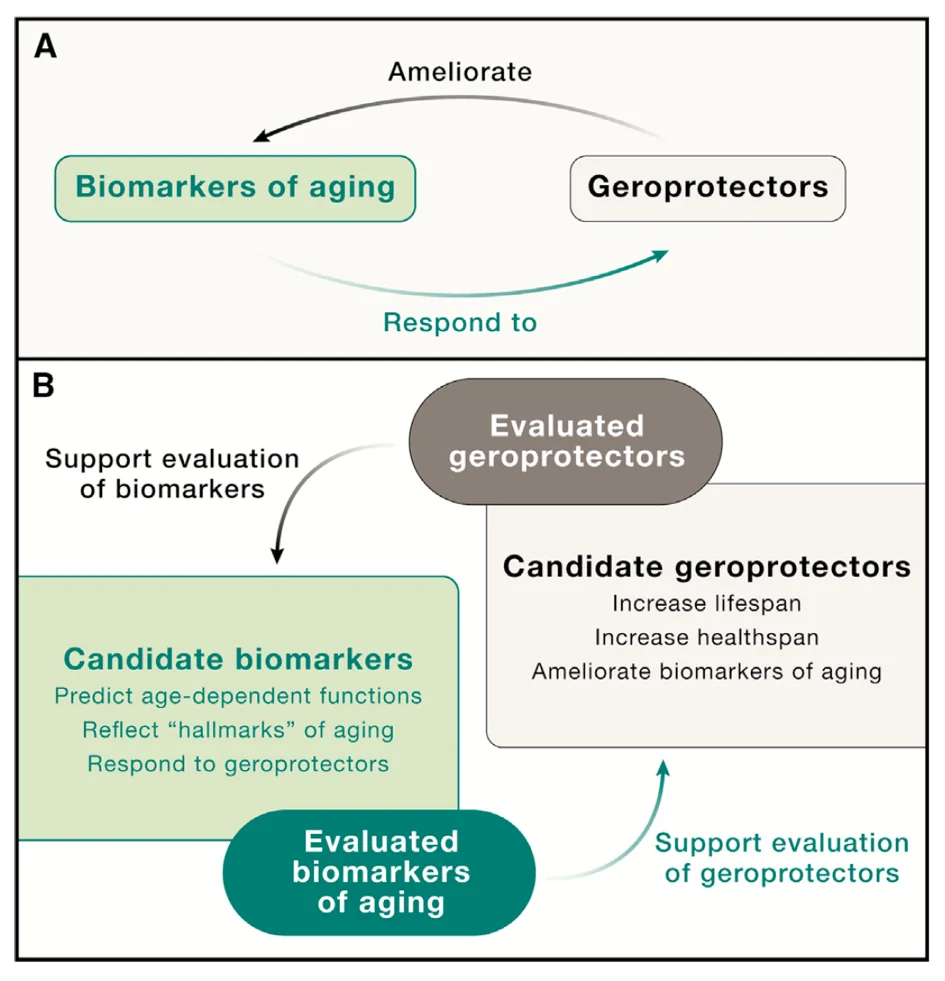
Some considerations on validation of biomarkers include the need to use them across multiple cohorts of humans, although such efforts might be hampered by the scarcity of available health data.
Conversely, several biomarkers rather than just one should probably be applied to the same cohort. In particular, the researchers suggest that for best results, biomarkers of aging, which they liken to odometers measuring total distance traveled, should be applied alongside biomarkers of the rate of aging (AgeDev) that act like speedometers. It seems that with both geroprotectors and biomarkers, combinations are key.
Biomarkers of aging could therefore serve several important roles in aging research: (1) to give an early indication of whether an intervention increases healthspan and/or lifespan; (2) to identify individuals who might benefit from a treatment; (3) to prioritize candidate interventions for longer-term assessment; and (4) as validated surrogate endpoints for regulatory and clinical purposes if short-term changes in aging biomarkers are shown to be predictive of longer-term outcomes. Our hope is that the consensus terminology, classification, evaluation criteria, and identified challenges and future directions for research presented in this work represent a solid first step toward achieving this goal.
Literature
[1] Moqri, M., Herzog, C., Poganik, J. R., Justice, J., Belsky, D. W., Higgins-Chen, A., … & Gladyshev, V. N. (2023). Biomarkers of aging for the identification and evaluation of longevity interventions. Cell, 186(18), 3758-3775.







