Using a novel senolytic drug, scientists have successfully eliminated senescent cells in human skin transplanted into mice. The treatment led to prolonged skin rejuvenation [1].
Senescence and senolytics
Senescent cells, also known as “zombie cells”, are cells that have stopped proliferating after being subjected to any of several types of stress (replicative, chemical, radiational, etc.) but evade clearance by the immune system. Such cells secrete the senescence-associated secretory phenotype (SASP), a cocktail of mostly harmful molecules that harm neighboring cells, driving more of them towards senescence and promoting inflammation. Cellular senescence is one of the hallmarks of aging.

Read More
Clearing away senescent cells with senolytics is a popular emerging strategy in the longevity field, but creating effective therapies have proved tricky. However, attempts continue, as evidenced by this new study coming from Japan.
Clearing out senescent fibroblasts in vitro
The researchers experimented with a recently discovered senolytic, which has a long chemical name shortened to BPTES. It works by inhibiting the enzyme glutaminase, which is essential for the survival of senescent cells [2]. Their goal was to see whether BPTES can effectively target senescent human skin cells in vitro and in vivo and whether clearing out those cells can lead to actual skin rejuvenation.
For their in vitro experiments, the researchers used human fibroblasts. Skin is thought to be one of the organs most affected by cellular senescence, with 15-60% of fibroblasts in the skin of aging mice being senescent [3]. Senescent cells are not always harmful. They play a role in wound healing [4], which is obviously important when it comes to skin, but with age, the “dark side” of senescent cells prevails.
The researchers induced senescence in the fibroblasts by three different method: replication, radiation, and treatment with doxorubicin, a chemotherapy drug. Such thoroughness is required, because senescence phenotypes differ significantly. The researchers then confirmed that some of the fibroblasts became senescent.
Here again, three different popular markers were used: senescence-associated ß-galactosidase (SA-ß-gal), p16, and p21. For additional robustness, senescence was also confirmed using morphological analysis, as senescent cells are generally larger and flatter than healthy cells. Another assay confirmed that proliferation levels in the culture had dropped as expected.
BPTES was used in different doses to eliminate the senescent fraction of the fibroblasts. The effect on the senescent cells was dose dependent. The highest dose decreased senescent cell viability almost to zero while barely affecting the viability of non-senescent cells, which shows both high efficacy and high specificity.
Rejuvenating human skin… in mice
To investigate the effect of BPTES on actual aged human skin, the researchers transplanted patches of it into naked mice. Prior to the transplantation, the samples were stained for senescence markers, which showed an abundance of senescent cells. The researchers then treated the transplants with BPTES and followed up for one month.
The treatment drastically reduced the number of cells that tested positive for SA-ß-gal, p16, and p21. Importantly, the levels of cellular senescence remained low even after a month of follow-up, indicating a protracted effect. The researchers also measured the proliferation marker Ki67 and saw a very significant increase in the rate of cell division following the treatment. BPTES also attenuated the levels of several SASP molecules that were elevated in the skin prior to transplantation (metalloproteinases and the inflammatory cytokines IL-1a, IL6, and TNFα).
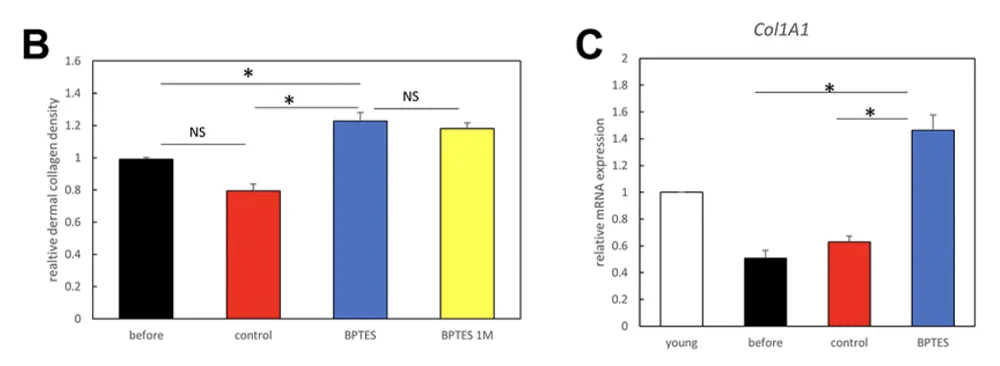
Collagen density, which determines skin elasticity and decreases with age, was significantly increased by the treatment, as shown by histological analysis. The mRNA expression of Col1A1, the gene that produces type I collagen, was more than twice as high in the treated transplants than in controls. Moreover, collagen levels remained high during the month-long follow-up. This suggests that senolysis might be a viable strategy for long-term skin rejuvenation.
Conclusion
In this study, the researchers were able to cause an impressive reduction of cellular senescence in human skin both in vitro and in vivo. The treatment had a protracted rejuvenative effect on aged human skin samples transplanted into mice. It is easy to see how this can lead to actual human trials in the near future.
Literature
[1] Takaya, K., Ishii, T., Asou, T., & Kishi, K. (2022). Glutaminase inhibitors rejuvenate human skin via clearance of senescent cells: a study using a mouse/human chimeric model. Aging, 14.
[2] Johmura, Y., Yamanaka, T., Omori, S., Wang, T. W., Sugiura, Y., Matsumoto, M., … & Nakanishi, M. (2021). Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science, 371(6526), 265-270.
[3] Wang, C., Jurk, D., Maddick, M., Nelson, G., Martin-Ruiz, C., & Von Zglinicki, T. (2009). DNA damage response and cellular senescence in tissues of aging mice. Aging cell, 8(3), 311-323.
[4] Wilkinson, H. N., & Hardman, M. J. (2020). Senescence in wound repair: emerging strategies to target chronic healing wounds. Frontiers in cell and developmental biology, 8, 773.



