A miniature review of clinical trials targeting aging was published in Frontiers in Aging by Dr. Morten Scheibye-Knudsen and colleagues [1]. This review specifically focuses on interventions that have shown strong clinical evidence that they impact aging.
Caloric restriction
We have previously discussed the effects of caloric restriction (CR) in reducing immunosenescence, improving DNA repair, and improving stem cell function in animal and cell studies. A couple of human studies have shown that CR reduces blood pressure, blood glucose levels, body weight, and resting metabolic rate [2,3]. Additionally, the Washington University CALERIE trial, a one-year study of 48 middle-aged overweight individuals, showed that caloric restriction improved insulin sensitivity and decreased fasting insulin; however, inflammation was not changed, as indicated by stable TNFa during caloric restriction [4]. In this same study, the CR group also had reduced levels of thyroid hormones T3 and T4. The review goes on to express other positive effects that caloric restriction has on metabolism and decreased inflammation.
Caloric restriction has also been shown to decrease unfavorable blood lipids and increase favorable lipids, reduce blood pressure and a decrease in C-reactive protein, an inflammation marker [5,6]. One study that further explored inflammation showed that specific inflammation markers were only impacted when IL-6 changes were significant [7].
Adiponectin, an important regulator of metabolism, was significantly increased in one study. Additionally, resting metabolic rate was decreased in all intervention groups but not in the control group [8]. Additional studies showed a 6% greater decrease in metabolic rate in CR invention groups compared to controls [3,9]. Regardless of the glycemic load of the CR diet, similar changes were seen in biomarkers of oxidative stress [10].
A recent trial showed that caloric restriction impacted healthspan but not the pace of aging [11]. The authors of this review mention that though results are promising within clinical settings, the evidence to show that these interventions work outside such settings is weak.
NAD+ supplements
We have previously published an article about NAD and its role in metabolism. Research suggests that NAD+ decreases with age and may be due to age-associated mitochondrial dysfunction [12]. NAD+ levels can be increased by consuming the biochemical precursors nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) [13]. Murine research has shown that these precursors improve physiological parameters and extend life [14,15].
A study in aged adult humans has shown that a combination of 250 mg and/or 500 mg NR and 50 mg and/or 100 mg pterostilbene increased blood NAD+ levels in the intervention groups in a dose-dependent manner. These supplements also improved liver enzyme biomarkers and decreased blood pressure; however, it could not be determined if it was pterostilbene, NR, or both that caused these benefits [16]. However, a study using 1000 and 200 mg doses of this combination, given two weeks prior to the injury, did not result in improved muscle injury recovery [17].
A 30-person study that examined kidney function, lipid profile, liver enzymes, hematology, and other biomarkers showed no difference between NR supplementation and placebo at 1000 mg for six weeks [18]. In a 12-person study, similar results were found in a 21-day trial [19]. This study also examined venous blood, urine, and skeletal muscle, showing significantly increased NAD+ levels, nicotinic acid adenine dinucleotide (NAAD), and NMN. This mini review goes on to explain other studies that show positive and negative results regarding NR.
A human study in 2018 showed no significant improvments in insulin sensitivity or other health parameters measured in a 12-week study of 2000 mg NR per day in 40 obese, insulin-resistant, sedentary men [20]. 1000 mg/day NR for 12 weeks also produced no significant changes in insulin resistance [21].
The authors note that research on the dosage of NAD+ increasing molecules in human studies is still in its early stages. They recommend longer and larger trials to further investigate the potential benefits of NAD+ increasing therapeutics.
Senolytics
Cellular senescence is one of the hallmarks of aging. A study in 14 patients with fibrosis showed improved physical benefits from dasatinib and quercetin in 6-minute walking distance, 4-minute gait speed and chair-stand time. However, they did not observe changes in pulmonary function, biomarkers, reported health, and frailty [22]. A study on 11 people with diabetes and kidney dysfunction showed that dasatinib and quercetin resulted in a significant decrease in senescent cell burden in adipose tissue along with senescence markers [23].
A recent trial of senolytics for knee osteoarthritis failed phase 2 trials. While the full results of these trials have not yet been published, there are many questions surrounding the trial design. The authors of this mini-review conclude this section by expressing the need for additional clinical trials involving senescence-reducing therapeutics.
Clinical trials that target mTOR
Promising data has been demonstrated that inhibiting mTOR extends lifespan in animals [24]. However, these results have not yet been reproduced in human trials that use rapamycin to inhibit mTOR [25,26]. Additionally, Dr. Mannick’s human research showed decreased infection rates and upregulated immune function in older adults [26]. A recent article shows how this work has led to industry collaboration on mTOR inhibition by mTOR analogs.
Rapamycin applied as a cream applied showed reduced skin senescence after 8 months of use [27]. Current proposed strategies to treat declining mitophagy include NAD+ supplements, activation of AMPK and/or SIRT1, and mTOR inhibition [28,29]. Urolithin A has been shown to improve in mitochondrial and muscle health [30], and we recently summarized a study showing that urolithin A affects mitochondrial and muscle in older adults. An animal study suggests that the mechanism involves mTOR inhibition [31].
Exercise and eating pattern
In long-term cross-country skiers, endurance training was associated with reduced systemic and muscle inflammation and improved telomeres [32,33]. A study done in 34 cyclist men at a variety of ages suggests that endurance training reduces inflammation, while senescence increases with age and may be unaffected by endurance training [34].
A different study of sleep, a plant-based diet, and exercise for 8 weeks showed a reduction of DNA methylation age by 3.32 years in 43 middle-aged and older males [35]. In a similar 2-year study with 219 females, DNA methylation was significantly decreased with a plant-based diet and exercise intervention [36]. Additionally, the authors of this review discuss how prior human data suggests that a Mediterranean diet reduces cardiovascular risk [37] by improving immune function and changing gut microbiome composition [38,39].
Conclusion
Beyond the scope of this review, the authors encouraged readers to become informed about other emerging inventions, such as senescence immunotherapy, stem cell reprogramming, nutraceutical inventions, and microbiome alterations.
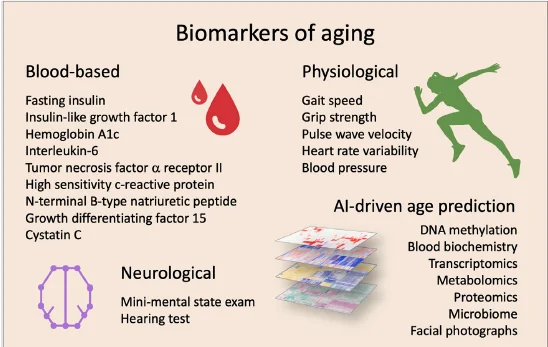
In their concluding paragraph, the authors discuss the need to carefully select biomarkers in clinical trials that target aging in humans. They further go on to explain how AI can help with age and health predictions. In the meantime, the authors include this figure of proposed biomarkers to target and/or monitor in clinical trials targeting aging.
Literature
[1] Nielsen, J. L., Bakula, D., & Scheibye-Knudsen, M. (2022). Clinical trials targeting aging. Frontiers in Aging, 3. https://doi.org/10.3389/fragi.2022.820215
[2] Velthuis-te Wierik, E. J., Hoogzaad, L. V., van den Berg, H., & Schaafsma, G. (1994). Effects of moderate energy restriction on physical performance and substrate utilization in non-obese men. International journal of sports medicine, 15(8), 478–484. https://doi.org/10.1055/s-2007-1021091
[3] Loft, S., Velthuis-te Wierik, E. J., van den Berg, H., & Poulsen, H. E. (1995). Energy restriction and oxidative DNA damage in humans. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 4(5), 515–519.
[4] Weiss, E. P., Racette, S. B., Villareal, D. T., Fontana, L., Steger-May, K., Schechtman, K. B., Klein, S., Holloszy, J. O., & Washington University School of Medicine CALERIE Group (2006). Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. The American journal of clinical nutrition, 84(5), 1033–1042. https://doi.org/10.1093/ajcn/84.5.1033
[5] Lefevre, M., Redman, L. M., Heilbronn, L. K., Smith, J. V., Martin, C. K., Rood, J. C., Greenway, F. L., Williamson, D. A., Smith, S. R., Ravussin, E., & Pennington CALERIE team (2009). Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis, 203(1), 206–213. https://doi.org/10.1016/j.atherosclerosis.2008.05.036
[6] Kraus, W. E., Bhapkar, M., Huffman, K. M., Pieper, C. F., Krupa Das, S., Redman, L. M., Villareal, D. T., Rochon, J., Roberts, S. B., Ravussin, E., Holloszy, J. O., Fontana, L., & CALERIE Investigators (2019). 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. The lancet. Diabetes & endocrinology, 7(9), 673–683. https://doi.org/10.1016/S2213-8587(19)30151-2
[7] Larson-Meyer, D. E., Newcomer, B. R., Heilbronn, L. K., Volaufova, J., Smith, S. R., Alfonso, A. J., Lefevre, M., Rood, J. C., Williamson, D. A., Ravussin, E., & Pennington CALERIE Team (2008). Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring, Md.), 16(6), 1355–1362. https://doi.org/10.1038/oby.2008.201
[8] Civitarese, A. E., Carling, S., Heilbronn, L. K., Hulver, M. H., Ukropcova, B., Deutsch, W. A., Smith, S. R., Ravussin, E., & CALERIE Pennington Team (2007). Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS medicine, 4(3), e76. https://doi.org/10.1371/journal.pmed.0040076
[9] Heilbronn, L. K., de Jonge, L., Frisard, M. I., DeLany, J. P., Larson-Meyer, D. E., Rood, J., Nguyen, T., Martin, C. K., Volaufova, J., Most, M. M., Greenway, F. L., Smith, S. R., Deutsch, W. A., Williamson, D. A., Ravussin, E., & Pennington CALERIE Team (2006). Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA, 295(13), 1539–1548. https://doi.org/10.1001/jama.295.13.1539
[10] Meydani, M., Das, S., Band, M., Epstein, S., & Roberts, S. (2011). The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE Trial of Human Caloric Restriction. The journal of nutrition, health & aging, 15(6), 456–460. https://doi.org/10.1007/s12603-011-0002-z
[11] Waziry, R., Corcoran, D. L., Huffman, K. M., Kobor, M. S., Kothari, M., Kraus, V. B., Kraus, W. E., Lin, D. T. S., Pieper, C. F., Ramaker, M. E., Bhapkar, M., Das, S. K., Ferrucci, L., Hastings, W. J., Kebbe, M., Parker, D. C., Racette, S. B., Shalev, I., Schilling, B., & Belsky, D. W. (2021). Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults: CALERIE™ Trial Analysis. https://doi.org/10.1101/2021.09.21.21263912
[12] Zhu, X. H., Lu, M., Lee, B. Y., Ugurbil, K., & Chen, W. (2015). In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proceedings of the National Academy of Sciences of the United States of America, 112(9), 2876–2881. https://doi.org/10.1073/pnas.1417921112
[13] Petr, M. A., Tulika, T., Carmona-Marin, L. M., & Scheibye-Knudsen, M. (2020). Protecting the aging genome. Trends in Cell Biology, 30(2), 117–132. https://doi.org/10.1016/j.tcb.2019.12.001
[14] Zhang, H., Ryu, D., Wu, Y., Gariani, K., Wang, X., Luan, P., D’Amico, D., Ropelle, E. R., Lutolf, M. P., Aebersold, R., Schoonjans, K., Menzies, K. J., & Auwerx, J. (2016). NAD? repletion improves mitochondrial and stem cell function and enhances life span in mice. Science (New York, N.Y.), 352(6292), 1436–1443. https://doi.org/10.1126/science.aaf2693
[15] Gomes, A. P., Price, N. L., Ling, A. J., Moslehi, J. J., Montgomery, M. K., Rajman, L., White, J. P., Teodoro, J. S., Wrann, C. D., Hubbard, B. P., Mercken, E. M., Palmeira, C. M., de Cabo, R., Rolo, A. P., Turner, N., Bell, E. L., & Sinclair, D. A. (2013). Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037
[16] Dellinger, R. W., Santos, S. R., Morris, M., Evans, M., Alminana, D., Guarente, L., & Marcotulli, E. (2017). Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ aging and mechanisms of disease, 3, 17. https://doi.org/10.1038/s41514-017-0016-9
[17] Jensen, J. B., Dollerup, O. L., Møller, A. B., Billeskov, T. B., Dalbram, E., Chubanava, S., Dellinger, R. W., Trost, K., Moritz, T., Ringgaard, S., Møller, N., Treebak, J. T., Farup, J., & Jessen, N. (2021). A randomized placebo-controlled clinical trial of nicotinamide riboside and Pterostilbene supplementation in experimental muscle injury in elderly subjects. https://doi.org/10.1101/2021.10.04.21264504
[18] Martens, C. R., Denman, B. A., Mazzo, M. R., Armstrong, M. L., Reisdorph, N., McQueen, M. B., Chonchol, M., & Seals, D. R. (2018). Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nature communications, 9(1), 1286. https://doi.org/10.1038/s41467-018-03421-7
[19] Elhassan, Y. S., Kluckova, K., Fletcher, R. S., Schmidt, M. S., Garten, A., Doig, C. L., Cartwright, D. M., Oakey, L., Burley, C. V., Jenkinson, N., Wilson, M., Lucas, S., Akerman, I., Seabright, A., Lai, Y. C., Tennant, D. A., Nightingale, P., Wallis, G. A., Manolopoulos, K. N., Brenner, C., … Lavery, G. G. (2019). Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell reports, 28(7), 1717–1728.e6. https://doi.org/10.1016/j.celrep.2019.07.043
[20] Dollerup, O. L., Christensen, B., Svart, M., Schmidt, M. S., Sulek, K., Ringgaard, S., Stødkilde-Jørgensen, H., Møller, N., Brenner, C., Treebak, J. T., & Jessen, N. (2018). A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. The American journal of clinical nutrition, 108(2), 343–353. https://doi.org/10.1093/ajcn/nqy132
[21] Dollerup, O. L., Chubanava, S., Agerholm, M., Søndergård, S. D., Altintas, A., Møller, A. B., Høyer, K. F., Ringgaard, S., Stødkilde-Jørgensen, H., Lavery, G. G., Barrès, R., Larsen, S., Prats, C., Jessen, N., & Treebak, J. T. (2020). Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. The Journal of physiology, 598(4), 731–754. https://doi.org/10.1113/JP278752
[22] Justice, J. N., Nambiar, A. M., Tchkonia, T., LeBrasseur, N. K., Pascual, R., Hashmi, S. K., Prata, L., Masternak, M. M., Kritchevsky, S. B., Musi, N., & Kirkland, J. L. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, 40, 554–563. https://doi.org/10.1016/j.ebiom.2018.12.052
[23] Hickson, L. J., Langhi Prata, L., Bobart, S. A., Evans, T. K., Giorgadze, N., Hashmi, S. K., Herrmann, S. M., Jensen, M. D., Jia, Q., Jordan, K. L., Kellogg, T. A., Khosla, S., Koerber, D. M., Lagnado, A. B., Lawson, D. K., LeBrasseur, N. K., Lerman, L. O., McDonald, K. M., McKenzie, T. J., Passos, J. F., … Kirkland, J. L. (2019). Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 47, 446–456. https://doi.org/10.1016/j.ebiom.2019.08.069
[24] ??Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., Nadon, N. L., Wilkinson, J. E., Frenkel, K., Carter, C. S., Pahor, M., Javors, M. A., Fernandez, E., & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392–395. https://doi.org/10.1038/nature08221
[25] Kraig, E., Linehan, L. A., Liang, H., Romo, T. Q., Liu, Q., Wu, Y., Benavides, A. D., Curiel, T. J., Javors, M. A., Musi, N., Chiodo, L., Koek, W., Gelfond, J., & Kellogg, D. L., Jr (2018). A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Experimental gerontology, 105, 53–69. https://doi.org/10.1016/j.exger.2017.12.026
[26] Mannick, J. B., Morris, M., Hockey, H. P., Roma, G., Beibel, M., Kulmatycki, K., Watkins, M., Shavlakadze, T., Zhou, W., Quinn, D., Glass, D. J., & Klickstein, L. B. (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Science translational medicine, 10(449), eaaq1564. https://doi.org/10.1126/scitranslmed.aaq1564
[27] Chung, C. L., Lawrence, I., Hoffman, M., Elgindi, D., Nadhan, K., Potnis, M., Jin, A., Sershon, C., Binnebose, R., Lorenzini, A., & Sell, C. (2019). Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. GeroScience, 41(6), 861–869. https://doi.org/10.1007/s11357-019-00113-y
[28] Fang, E. F., Scheibye-Knudsen, M., Brace, L. E., Kassahun, H., SenGupta, T., Nilsen, H., Mitchell, J. R., Croteau, D. L., & Bohr, V. A. (2014). Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell, 157(4), 882–896. https://doi.org/10.1016/j.cell.2014.03.026
[29] Scheibye-Knudsen, M., Mitchell, S. J., Fang, E. F., Iyama, T., Ward, T., Wang, J., Dunn, C. A., Singh, N., Veith, S., Hasan-Olive, M. M., Mangerich, A., Wilson, M. A., Mattson, M. P., Bergersen, L. H., Cogger, V. C., Warren, A., Le Couteur, D. G., Moaddel, R., Wilson, D. M., 3rd, Croteau, D. L., … Bohr, V. A. (2014). A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell metabolism, 20(5), 840–855. https://doi.org/10.1016/j.cmet.2014.10.005
[30] Andreux, P. A., Blanco-Bose, W., Ryu, D., Burdet, F., Ibberson, M., Aebischer, P., Auwerx, J., Singh, A., & Rinsch, C. (2019). The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nature metabolism, 1(6), 595–603. https://doi.org/10.1038/s42255-019-0073-4
[31] Ryu, D., Mouchiroud, L., Andreux, P. A., Katsyuba, E., Moullan, N., Nicolet-Dit-Félix, A. A., Williams, E. G., Jha, P., Lo Sasso, G., Huzard, D., Aebischer, P., Sandi, C., Rinsch, C., & Auwerx, J. (2016). Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature medicine, 22(8), 879–888. https://doi.org/10.1038/nm.4132
[32] Beavers, K. M., Brinkley, T. E., & Nicklas, B. J. (2010). Effect of exercise training on chronic inflammation. Clinica chimica acta; international journal of clinical chemistry, 411(11-12), 785–793. https://doi.org/10.1016/j.cca.2010.02.069
[33] Østhus, I. B., Sgura, A., Berardinelli, F., Alsnes, I. V., Brønstad, E., Rehn, T., Støbakk, P. K., Hatle, H., Wisløff, U., & Nauman, J. (2012). Telomere length and long-term endurance exercise: does exercise training affect biological age? A pilot study. PloS one, 7(12), e52769. https://doi.org/10.1371/journal.pone.0052769
[34] Balan, E., De Groote, E., Bouillon, M., Viceconte, N., Mahieu, M., Naslain, D., Nielens, H., Decottignies, A., & Deldicque, L. (2020). No effect of the endurance training status on senescence despite reduced inflammation in skeletal muscle of older individuals. American journal of physiology. Endocrinology and metabolism, 319(2), E447–E454. https://doi.org/10.1152/ajpendo.00149.2020
[35] Fitzgerald, K. N., Hodges, R., Hanes, D., Stack, E., Cheishvili, D., Szyf, M., Henkel, J., Twedt, M. W., Giannopoulou, D., Herdell, J., Logan, S., & Bradley, R. (2021). Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging, 13(7), 9419–9432. https://doi.org/10.18632/aging.202913
[36] Fiorito, G., Caini, S., Palli, D., Bendinelli, B., Saieva, C., Ermini, I., Valentini, V., Assedi, M., Rizzolo, P., Ambrogetti, D., Ottini, L., & Masala, G. (2021). DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: the DAMA study. Aging cell, 20(10), e13439. https://doi.org/10.1111/acel.13439
[37] Estruch, R., Ros, E., Salas-Salvadó, J., Covas, M. I., Corella, D., Arós, F., Gómez-Gracia, E., Ruiz-Gutiérrez, V., Fiol, M., Lapetra, J., Lamuela-Raventos, R. M., Serra-Majem, L., Pintó, X., Basora, J., Muñoz, M. A., Sorlí, J. V., Martínez, J. A., Fitó, M., Gea, A., Hernán, M. A., … PREDIMED Study Investigators (2018). Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. The New England journal of medicine, 378(25), e34. https://doi.org/10.1056/NEJMoa1800389
[38] Maijo, M., Ivory, K., Clements, S. J., Dainty, J. R., Jennings, A., Gillings, R., Fairweather-Tait, S., Gulisano, M., Santoro, A., Franceschi, C., Carding, S. R., & Nicoletti, C. (2018). One-Year Consumption of a Mediterranean-Like Dietary Pattern With Vitamin D3 Supplements Induced Small Scale but Extensive Changes of Immune Cell Phenotype, Co-receptor Expression and Innate Immune Responses in Healthy Elderly Subjects: Results From the United Kingdom Arm of the NU-AGE Trial. Frontiers in physiology, 9, 997. https://doi.org/10.3389/fphys.2018.00997
[39] Ghosh, T. S., Rampelli, S., Jeffery, I. B., Santoro, A., Neto, M., Capri, M., Giampieri, E., Jennings, A., Candela, M., Turroni, S., Zoetendal, E. G., Hermes, G., Elodie, C., Meunier, N., Brugere, C. M., Pujos-Guillot, E., Berendsen, A. M., De Groot, L., Feskins, E., Kaluza, J., … O’Toole, P. W. (2020). Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut, 69(7), 1218–1228. https://doi.org/10.1136/gutjnl-2019-319654



