Clinical Trial of Nicotinamide Riboside Completed

Today, we want to highlight a recent human trial of the popular supplement nicotinamide riboside, a compound that has been shown in mice to restore NAD+ levels. The compound has had impressive results against some aspects of aging in mouse studies, and there is now some more data for NR in humans [1].
What is nicotinamide riboside?
Nicotinamide adenine dinucleotide (NAD+) is a chemical that facilitates the production of energy from sugar and is present in every cell in our body. As well as being important in energy production, it is also involved in DNA repair, cellular signaling, and many other cell functions.
Unfortunately, as we age, the availability of NAD+ declines in the body, and this appears to support the development of metabolic disorders and other age-related diseases. It is involved in many systems in the body, from useful ones, such as DNA repair, to potentially harmful ones, such as inflammation caused by senescent cells. Due to this, it is currently unknown if increasing NAD+ signaling will cause more harm than good – more work, such as the study being reported, must be done before we have an answer.
Three pathways, one molecule
Given how critical NAD+ is to cellular function, our cells have a number of ways to obtain it; this redundancy in the system is likely to be due to how important NAD+ is.
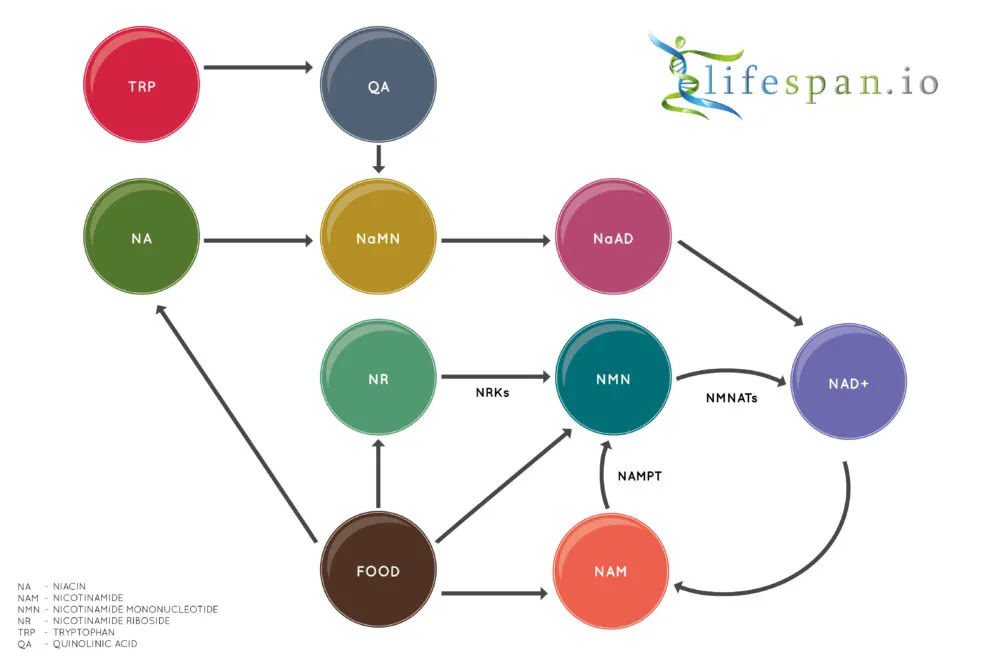
NAD+ can be created de novo, going through multiple enzymatic steps in the de novo pathway (kynurenine pathway), ultimately producing nicotinic acid mononucleotide (NaMN) as the final step in this process. The term “de novo” means that one biomolecule, in this case, NAD+, is produced anew from a different molecule. Essentially, the NaMN molecule is built from scratch, starting with the essential amino acid L-tryptophan (Trp). The de novo pathway is the only non-vitamin B3-based pathway that allows the creation of NAD+.
Niacins, such as nicotinic acid- and niacinamide-containing compounds, are taken in via dietary sources or supplements and can be used to create NAD+, with each form entering the system at different points. Nicotinic acid (NA) and nicotinic acid riboside (NAR) produce NAD+ via the Preiss-Handler pathway. This pathway begins with NA or NAR and converts both into NAD+ via a series of enzymatic steps. As this diagram shows, NaNM is an intermediate in the pathway, meaning that the de novo pathway shares several common enzymatic steps with the Preiss-Handler pathway on the road to NAD+ creation.
The final pathway is the salvage pathway, which converts niacinamide (NAM) into NAD+ through a series of steps that continually cycle and recover the NAD+ once it has been used by the cell, turning it back into NAM to create NAD+ again; hence, it is called the salvage pathway. Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) also both feed into the salvage pathway and are converted into NAD+.
Nicotinamide phosphoribosyltransferase (NAMPT) is also a precursor for NAD+ and part of the salvage pathway and, as recent research shows, can also influence lifespan when levels are increased in mice.
Putting NR to the test
Researchers have recently published the results of a small-scale human trial using the NAD+ precursor NR; however, this study has not yet been subject to peer review and is pending publication in a journal. The NR for the study was supplied by Chromadex, the patent holder and sole producer of Niagen, its particular form of NR; Dr. Charles Brenner, one of the leading study authors, declared that he holds stock in this company. This is not necessarily a red flag, nor does it invalidate the study, but it is something to keep in mind.
This study found no correlation between the age of patients and the level of NAD+ in muscle and brain tissue. Therefore, we might not expect age-related changes in these tissues to be reversed due to supplementation of an NAD+ raising supplement, such as nicotinamide riboside.
In addition, the study layout was flawed, which may further harm efforts to detect an effect from the supplement. A low number of patients in both treatment and comparison groups, combined with a large range of patient weights (ranging from the lower ‘healthy’ weights to an upper ‘overweight’ limit) and a flawed method of measuring this weight (BMI, which can be distorted by patient height and other factors), reduce the accuracy of any results given in this study.
Furthermore, with multiple targets to study, the threshold of certainty below which we could say that the drug is effective is significantly lowered. This was not mentioned by the study authors.
The study found no clinical benefit to supplementation with the drug
During the study, the impact of the drug on a few markers of disease and frailty was analyzed – specifically, grip strength (no significant effect), muscle blood flow and metabolism (no significant effect), and meNAM, a protein thought to be increased in type 2 diabetes and insulin resistance (a significant increase, although the study did not find a significant impact on type 2 diabetes or insulin resistance).
While this may be discouraging, this is still a study with low numbers of patients, which means that we might expect this result even if the drug works, although the significant rise in diabetes-causing meNAM is concerning.
The study did find significant effects on some protein factors
The study found that the level of a protein called NAAD – a sensitive biomarker common to NAD+ increasing supplements [2] – was very significantly raised in muscle by nicotinamide riboside, even taking into account the failure of the study to account for the number of variables it was testing. At a similar level of significance, the study found an increase in NAM excretion products – which the authors suggested could be a result of the target tissues already having enough NAD+ before the study began.
A significant effect of the drug was found on some markers of inflammation (such as IL-2, IL-5 and IL-6) as well, reducing their activity – and since chronic inflammation is a major driver of aging, this could be a promising sign of the drug having an effect on this aspect of aging, keeping in mind that this significance may not have been found in a study with sufficiently strict significance boundaries (although the impact on IL2 was particularly significant). Other inflammatory markers, such as TNFalpha, gave conflicting results, while five other markers showed no significant impact of the drug.
NAD+ is modulated by conditions of metabolic stress and has been reported to decline with aging, but human data are sparse. Nicotinamide riboside (NR) supplementation ameliorates metabolic dysfunction in rodents. We aimed to establish whether oral NR supplementation in aged participants can increase the skeletal muscle NAD+ metabolome, and questioned if tissue NAD+ levels are depressed with aging. We supplemented 12 aged men with NR 1g per day for 21-days in a placebo-controlled, randomized, double-blind, crossover trial. Targeted metabolomics showed that NR elevated the muscle NAD+ metabolome, evident by increased nicotinic acid adenine dinucleotide and nicotinamide clearance products. Muscle RNA sequencing revealed NR-mediated downregulation of energy metabolism and mitochondria pathways. NR also depressed levels of circulating inflammatory cytokines. In an additional study, P magnetic resonance spectroscopy-based NAD+ measurement in muscle and brain showed no difference between young and aged individuals. Our data establish that oral NR is available to aged human muscle and identify anti-inflammatory effects of NR, while suggesting that NAD+ decline is not associated with chronological aging per se in human muscle or brain.
Conclusion
Although the flawed setup of the study – and the low number of patients – limits our confidence in the results of the study, no toxicity was found. While the study detected no functional improvements resulting from the use of the drug, this could have been due to a number of confounding factors or caused by the small number of patients enrolled in the study. Given these results, it seems that further research is needed to come to any conclusions about the effectiveness of nicotinamide riboside.
Literature
[1] Elhassan, Y. S., Kluckova, K., Fletcher, R. S., Schmidt, M., Garten, A., Doig, C. L., … & Wilson, M. (2019). Nicotinamide riboside augments the human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures in aged subjects: a placebo-controlled, randomized trial. BioRxiv, 680462.
[2] Trammell, S. A., Schmidt, M. S., Weidemann, B. J., Redpath, P., Jaksch, F., Dellinger, R. W., … & Brenner, C. (2016). Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nature communications, 7, 12948.







