For the 11th Year in Copenhagen: Highlights from ARDD 2024
- We attended the biggest conference in the longevity field.

The Copenhagen-based Aging Research and Drug Discovery Meeting (ARDD) was already enormous. Last year, there were well over a thousand physical participants, and many more online, attending five days of wall-to-wall talks: more than a hundred in total. We called it “the mother of all longevity conferences.” At the dawn of its second decade, ARDD’s attendance of researchers, entrepreneurs, and enthusiasts grew even bigger and was sold out even sooner, solidifying its iconic status in the field.
While the venue and the duration remained the same, not that there’s anything wrong with the beautifully decorated and centrally located Copenhagen University’s Museumhuset, the expansion was achieved by adding tracks: Longevity Medicine Track & XPRIZE Healthspan Team Summit, Emerging Tech Workshop, and Physics in Aging Biology. As a result, the overall number of talks shot up to about 160.
A few times, this made me desperately want to have that clock that allowed Hermione to attend multiple Hogwarts classes simultaneously. Unfortunately, we can’t turn the clock back literally, but we hope to eventually be able to do this figuratively, by reversing aging, which is, of course, what the conference was about.
As usual, we are only able to bring you a fraction of the talks (in ARDD’s case, a particularly small fraction). We apologize to all the amazing speakers who didn’t make the cut.
The case for bioidentical hormones
Longevity medicine, traditionally well-represented at ARDD, took an even more prominent place this time. The first day of the conference saw a number of appearances, including by the first lady of Thailand, Pakpilai Thavisin.
Pakpilai, a medical doctor, talked about the numerous health problems associated with menopause, such as mood and sleep disorders. However, there is evidence that harm from menopause goes far beyond that. For instance, premenopausal women have lower incidences of hypertension and other cardiovascular events than men, but those differences diminish after menopause, hinting at a potentially protective role of estrogen. “If we want to cure aging,” Pakpilai asked, “why not cure menopause?”
It’s not just about dwindling estrogen levels. According to Pakpilai, all hormones decline with age. “Our biological clock tells us ‘to shrink and die’ as we age,” she explained.
However, hormone replacement therapy (HRT) got a bad rap due to associations with cancer. Estrogen alone, Pakpilai said, indeed increases the risk of endometrial cancer. This is mitigated by adding progesterone, but this combo has been linked to an increased risk of breast cancer.
Pakpilai argued that the problem was non-bioidentical hormones – synthetic molecules that are similar but not identical to human hormones. Those molecules are produced by pharma companies because they can be patented, unlike bioidentical ones. The latter can be derived from various natural sources and are associated with fewer side effects and health risks.
Pakpilai stressed the need to find a business model for producing bioidentical hormones for HRT and called for a wider acceptance of HRT based on those hormones for postmenopausal women.

More longevity medicine
Other speakers highlighted the successes and challenges of today’s longevity medicine. Most of those challenges have remained the same since last year and include a lack of proven anti-aging therapies. Nevertheless, longevity clinics are relevant due to their proactive/preventative approach not seen in traditional medicine, extensive testing, and creative mixing of therapies that have been documented to be geroprotective, such as diet, exercise, repurposed drugs, and various supplements.
Evelyne Bischof, a pioneer of longevity medicine, reminded the audience that back in 2019, she, Alex Zhavoronkov of InSilico, and several others found the Longevity Education Hub. The hub features a basic longevity medicine course, an advanced one, and a third course on investing in longevity biotech, all of which are free to take. The medicine courses were translated into several languages by volunteers. Today, the enrollment stands at about 7,000.
However, the big news was that for the first time, the curriculum has been implemented in an academic institution. In one of Indonesia’s universities, it became an obligatory course for medical doctors, and several other countries are currently on the way to adopting it, according to Evelyne.
Alex Zhavoronkov, ARDD co-founder, also stressed the importance of longevity medicine, saying that this industry “cannot move faster than physicians.”
Aging and salmon suicide
Michael Ringel of Boston Consulting Group gave a deep and fascinating talk on the evolutionary origins of aging. Geroscientists are mostly focused on how aging happens, but why it happens is also important.
Several theories have been proposed. According to Ringel, those explanations can be divided into three broad categories: Mechanistic, Weakening Selection, and Optimization. The first one posits that aging happens due to the inability of evolution to eliminate physical constraints such as the damage that arises from normal biological processes. Basically, miracles don’t happen.
The second one means that, as organisms age and survival declines, there is less evolutionary pressure to maintain the traits that would keep them healthy later in life. Selection becomes so weak that random mutations, including those that accelerate aging or cause diseases, are no longer removed effectively from the gene pool. This allows aging and late-life deterioration to persist in the population.
Finally, the optimization paradigm says, in the words of one paper, that “nature strikes a balance in allocating resources to growth vs. maintenance vs. reproduction.” What is optimized is reproduction fitness: the “long-run rate of increase of a line of descent”, which includes survival, fecundity (the number of offspring), generation time, and quality of those offspring. That’s all evolution is concerned with rather than the survival of one organism.
Michael argued that current empirical evidence is best explained by the optimization paradigm. This has an important implication: a vast majority of pro-longevity mutations, just like other mutations, are a step away from that carefully optimized state. While evidence exists that “you can get longevity without fecundity trade-off,” Michael claims that if you look at a broader context of reproductive fitness, which stretches beyond fecundity, you will probably find how it is hurt by the mutation.
For instance, salmon’s seemingly aimless self-destructive behavior makes sense when you look at offspring fitness: adult salmon’s carcasses are food, essential for offspring survival in nutrient-poor streams. However, in a nutrient-rich environment, salmon can quickly develop the ability to spawn.
Optimization theory makes a testable prediction that lifespan-increasing mutations generally reduce fitness. This can be important for numerous aspects of geroscience, such as translation and drug development.

A peptide that could
Some longevity biotechs don’t shy from making big claims, and Maxwell Biosciences is one of them. Its goal is to create “a synthetic immune system” that would give us wide protection against microbial pathogens.
Maxwell’s founder and CEO, J. “Scotch” McClure, stressed the importance of the microbiome for most, if not all, hallmarks of aging. For instance, dysbiosis affects mitochondrial health, which is evident in chronic kidney diseases and in HIV. Bacterial and viral factors have also been implicated in genomic instability, dysregulated nutrient sensing, telomere attrition, and other problems.
Those microbial pathogens present unique challenges. Bacteria and viruses evolve quickly, developing drug resistance. Fungi can wreak a lot of havoc and are understudied. The body has defense mechanisms, but they dwindle and get overwhelmed as we age.
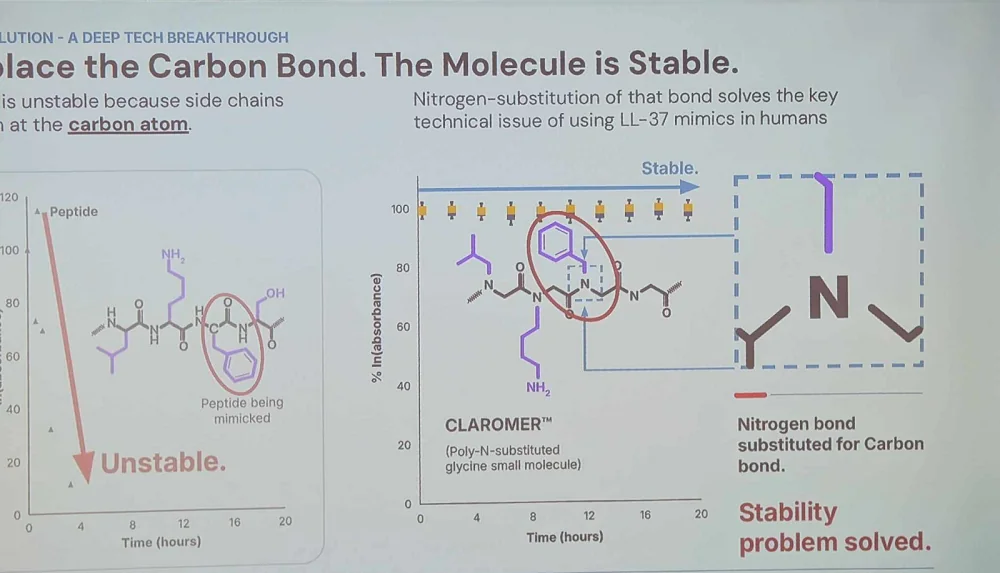
One such mechanism is the antimicrobial peptide LL-37. According to McClure, it’s potent against virtually all types of microbial pathogens but is rapidly degraded by proteasomes that target the carbon bond. Maxwell’s answer is to replace that carbon bond with a more stable nitrogen bond. The resulting product “captures all the innate virucidal, bactericidal, fungicidal, and anti-aging benefits of natural LL-37 in a stable form optimized for humans.”
Maxwell’s LL-37-mimicking candidate kills viruses and bacteria by permeating their membranes and is effective even against highly resistant bacterial strains because its “membrane-targeting mechanism of action cannot be circumvented by current or feasible bacterial resistance mechanisms.” It’s also effective against fungi.
Maxwell runs several high-profile collaborations and is wrapping up a study in rhesus macaques with results expected later this month. Human clinical trials will begin next year. If all this sounds too good to be true, there is a caveat: a study linked LL-37 to Alzheimer’s disease progression.
The long-overdue Alzheimer’s breakthrough?
This ARDD saw increased attention from locals, including opening speeches by Henrik Wegener, Rector of the University of Copenhagen, and Mette Kierkgaard, Danish Minister for Senior Citizens. Some Danish companies were also represented, such as the international pharma company H. Lundbeck A/S (commonly known simply as Lundbeck). Johan Luthman, head of R&D, gave a talk centered on brain aging, one of Lundbeck’s key areas of interest.
The symptoms of brain aging include decreased cerebral blood flow, brain volume shrinkage, and brain lesions, such as hyperintensities and microbleeds. While in most brains, it only results in slowly deteriorating function (although some functions actually get enhanced), in some, brain aging can speed up and progress towards brain frailty and sickness.
While many neurodegenerative diseases have strong ties to family history, aging is still the greatest risk factor. Bad sleep quality also correlates substantially with brain health. Johan’s point was that neurodegeneration can be reversed, putting the brain back on track for “healthy” brain aging.
One of the main functions that are disrupted in brain aging, leading to neurodegeneration, is what Johan called “brain washing” – the action of the glymphatic system. This is a network of channels in the brain that allows cerebrospinal fluid (CSF) to flush out waste, including proteins like beta-amyloid and tau, which are associated with neurodegenerative diseases. The glymphatic system is most active during deep sleep.
Johan then gave a review of new anti-Alzheimer’s drugs. After two decades of failures, he claimed, there’s finally a breakthrough in the field, with Leqembi being the first ever drug to receive full US approval as a disease-modifying treatment in AD. Leqembi works by stimulating the glymphatic system, just like another novel drug, Kisunla (Leqembi was approved in 2023, and Kisunla in 2024).
The third drug, Rexulti, is the first ever US-approved treatment for behavioral and psychological symptoms of dementia (BPSD). The new drug treats Alzheimer’s-related agitation. Often overlooked, this symptom is “what drives people into nursing homes,” Johan said.
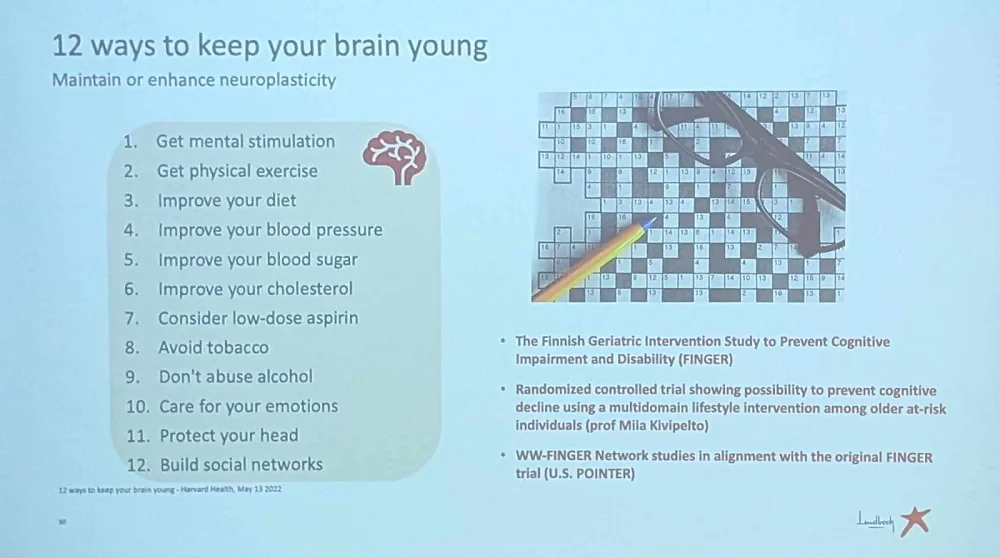
Johan is optimistic about the future of anti-dementia therapies. He pointed at the recent changes in regulation that favor their approval, including acceptance of new biomarkers such as PET imaging as a marker of amyloid pathology, and neurofilament light chain (NfL) as a fluid biomarker of neurodegeneration. Meanwhile, people can keep their brains healthier using these tips from Johan:
In sickness and in health
InSilico, the company co-founded by Alex Zhavoronkov, was widely represented at the conference. Alex himself gave a talk describing the progress the company has made over the past year.
Interestingly, he did not start with aging biology. Instead, he touted InSilico’s and ARDD’s focus on sustainability. This year, the conference organizers decided to use some of the proceedings to plant 10,000 trees. Alex also said that the technology for small molecule discovery his company is developing can be used for carbon capture, and InSilico is already profiting from selling their software to the companies in the field. You can sign ARDD’s Copenhagen Longevity and Sustainability Declaration here.
InSilico, an AI-centered company that created a software suit for drug discovery automation, prides itself on discovering targets and drug candidates and moving them through the pipeline at lightning speeds. The average time of developing a preclinical candidate (PCC) is 13 months, and the company currently has 18 PCCs nominated. Nine of them are in human clinical trials, including two in Phase 2.
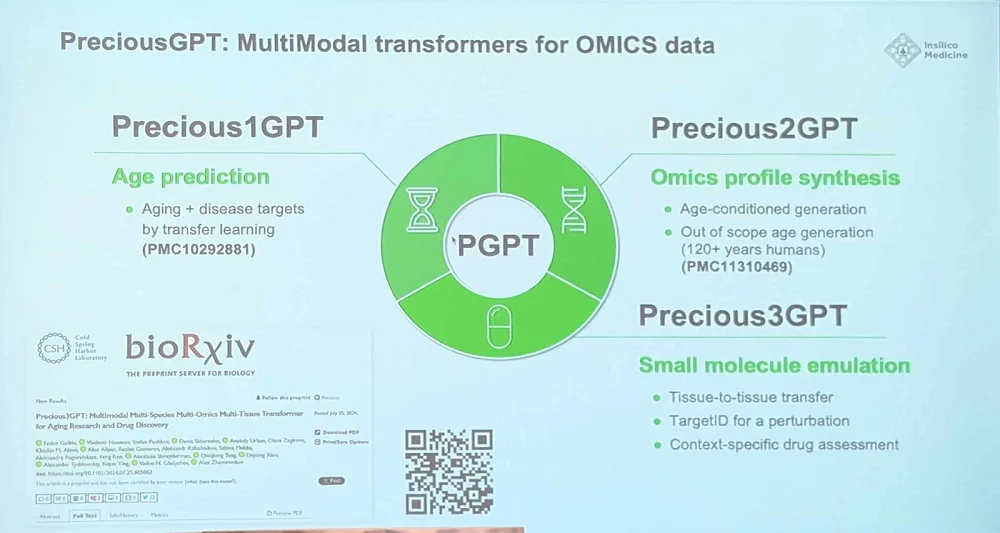
Another big project is creating a multimodal transformer AI model for aging. This idea was first voiced by Alex two years ago, and now the model lineup includes Precious1GPT (“a transformer-based model with aging clock functionality for aging-related pathology research”), Precious2GPT (“a compound model combining a transformer and a diffusion architectures with omic data generation capabilities”), and Precious3GPT (“a genuinely multimodal transformer-based model trained to emulate the workflow of case-control studies, with an emphasis on chemical perturbations.”) This versatile model can feed on multiple data types and perform multiple tasks such as small molecule emulation. The name was chosen to reflect the versatility and the ambition (“One model to rule them all”, Alex said).
In probably the most interesting part of the talk, Alex told the audience how he was amazed to find that between the genes linked to aging by multi-species, multi-tissue mammalian epigenetic clocks were several of the targets their model had proposed, including TNIK, in which Alex places a lot of hopes.
According to Alex, analyzing CpG cites and linking them to targetable genes is a promising novel discovery route, and it shows that “you can derive useful therapeutic targets from aging research, which is the entire point of this conference.” Pharma is opening up to aging research, Alex said, and this kind of studies gives them more evidence.
Very rarely do longevity conferences provide decent gossip fodder, but this ARDD was an enormous exception. In an unexpected grand finale to his talk, Alex proposed on stage to Dominika Wilczok, a longevity activist and researcher. Lifespan.io congratulates the couple and wishes them an infinite lifetime of love, happiness, curiosity, and discovery.

TNIK, TNIK everywhere
Another local, Lykke Sylow of the University of Copenhagen, talked about her research that probably made Alex even happier. Lykke’s team is looking for targets that can be used to alleviate muscle aging and atrophy.
According to Lykke, the obesity epidemic should be called “the sarcopenic obesity epidemic,” because obesity is often accompanied by skeletal muscle loss. While people can lose weight, including with the latest and greatest weight loss drugs such as GLP-1 receptor agonists, they are also poised to lose a lot of muscle mass in the process: about 40% on average of the total weight loss.
This muscle mass loss, as well as the rebound in body weight after intervention cessation, can be partially mitigated by exercise. However, exercise has low levels of adherence and might not be available to everyone. Lykke called exercise “an old trick” and said they were looking for new ones: interventions that would mimic the effects of exercise.
Several companies in the field are already working on this, including BioAge. Lykke also mentioned Eli Lilly’s acquisition of Versanis, manufacturer of bimagrumab, in order to combine this drug with the weight loss drug tirzepatide.
One of the targets that Lykke’s team identified is the crucial molecule TNIK. Knocking out msn, the TNIK ortholog in flies, made them metabolically inflexible: unable to switch energy sources between fat, glucose, and protein. Metabolic flexibility is also impaired in aging.
The group then created TNIK-knockout mice and placed them either on a regular or on high fat / high sucrose diet, which mimics a standard “Western” diet. The knokout mice were resistant to diet-induced obesity, despite consuming the same or greater number of calories. Yet, they retained their lean mass and showed increased energy expenditure and activity.
TNIK deficiency also rescued glucose tolerance and insulin sensitivity in mice on a high-fat/high-fructose diet by improving insulin-stimulated glucose uptake and prevented hepatic steatosis. Finally, the group found that TNIK variants in humans correlate with obesity and diabetes-related traits.

Reining transposons in
John Sedivy of Brown University reminded the audience that about half of our genome consists of repeated sequences, mostly transposons associated with viruses. While some transposons are benign “viral fossils” that lost their ability to replicate, a majority can still do it if the patches of the chromatin where they are located are derepressed.
Transposon reactivation increases with age and has been linked to multiple age-related conditions. This can happen in a positive feedback loop: cellular senescence leads to chromatin opening, LINE-1 (the most ubiquitous retrotransposon) derepression, antiviral response, and then to chronic inflammation.
How do retrotransposons procreate? When their DNA is derepressed and transcribed into RNA, the enzyme reverse transcriptase transcribes this RNA back into DNA, which is called complementary DNA (cDNA). These cDNA strands can then insert themselves into other parts of the genome (that’s how humans and many other species ended up with huge chunks of their DNA consisting of nothing but retrotransposons). However, cDNA is recognized by the CGAS/STING pathway, which triggers an inflammatory response.
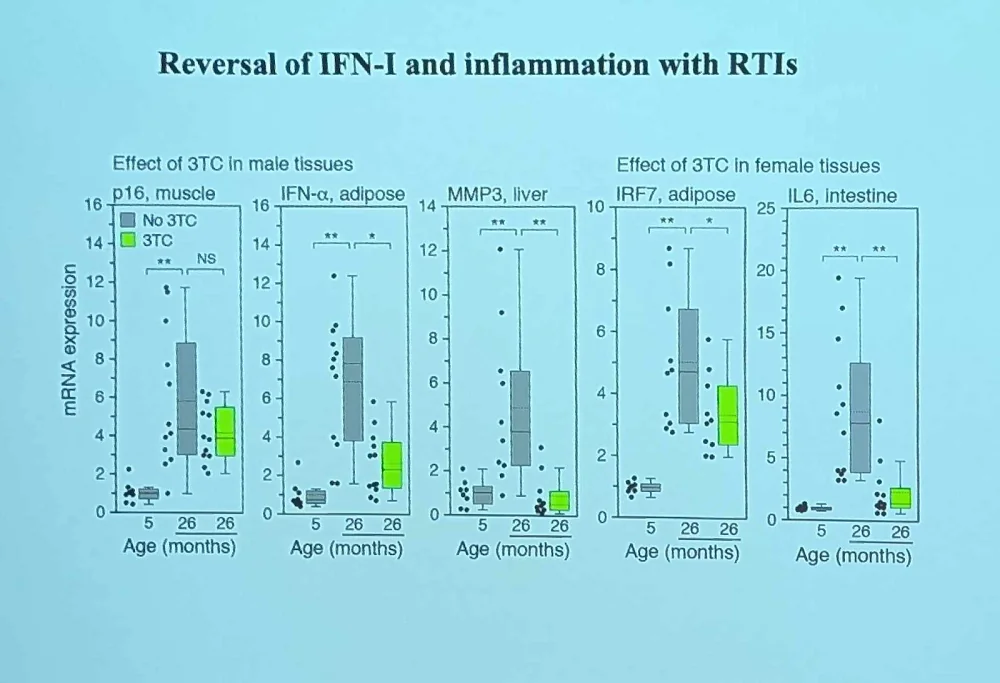
Transposon Therapeutics, the company that John advises, is built on the idea that we can use existing reverse transcriptase inhibitors (such as anti-HIV drugs) against age-related retrotransposon activation. Studies in animal models show that these drugs can have a massive effect on inflammation, cellular senescence, and age-related cognitive decline.
The company is already deep in clinical trials with censavudine. This reverse transcriptase inhibitor was discovered at Yale, licensed to GSK to market as an HIV drug, and then licensed by Transposon, which also acquired all the NID-enabling data.
According to Jogn, censavudine proved to be highly potent and bioavailable, demonstrating low off-target binding and good brain penetration. The company’s main indication is progressive supranuclear palsy (PSP), although it also eyes amyotrophic lateral sclerosis (ALS). The candidate drug TPN-101 produced large reductions in neurofilament light chain and the pro-inflammatory cytokine IL-6.
New is the well-forgotten old
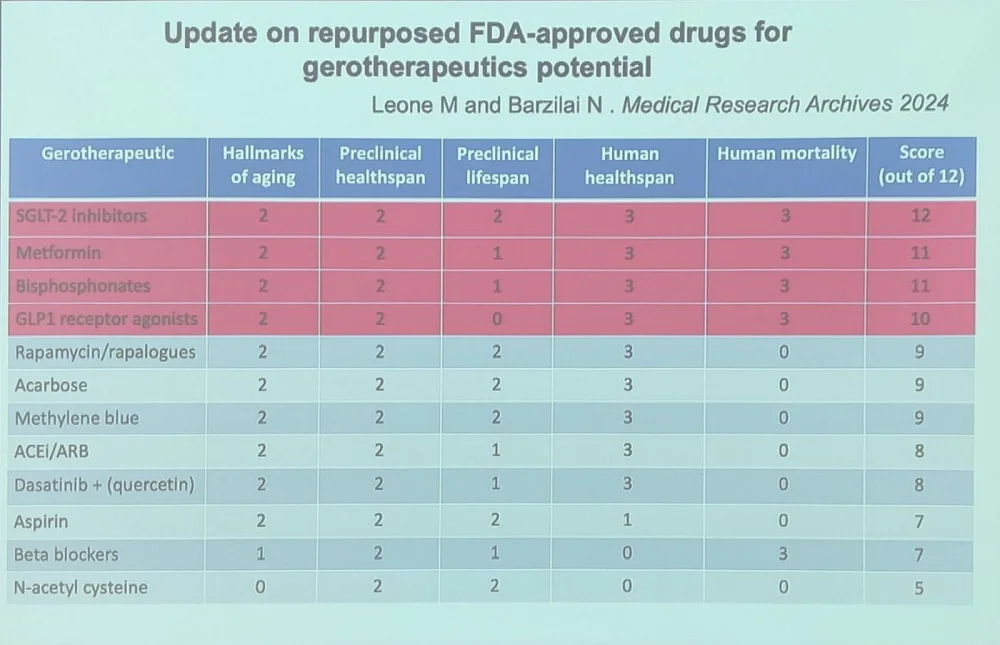
The renowned geroscientist Nir Barzilai of Albert Einstein College of Medicine presented a new paper he co-authored with Michael Leone, which is an update of an earlier study on the same topic. The paper compiles data on possible anti-aging effects of FDA-approved drugs.
Nir has argued for years for using existing drugs as possible geroprotectors and is known as a big proponent of metformin. Nir conceived the widely anticipated Targeting Aging with Metformin (TAME) trial, which has yet to take off the ground.
In the new paper, 12 drugs or drug classes were ranked by several criteria: the number of hallmarks of aging they are currently known to hit, as well as their effects on healthspan and lifespan in preclinical models, human healthspan, and human mortality.
Anti-diabetes medications – SGLT2 inhibitors and metformin – took the crown. The former include drugs such as canagliflozin, which have been recently shown to extend lifespan in animal models. However, these two types of drugs lead mostly because of human data, which is to be expected: both have been prescribed to chronically ill patients for many years and hence are well-studied in the context of human mortality.
Bisphosphonates are a class of drugs used to treat osteoporosis and other bone conditions. According to Nir, they significantly lower ICU mortality. The fourth place is taken by GLP1 receptor agonists which include popular novel weight loss drugs such as semaglutide. Recent data suggests that in addition to reversing obesity, those drugs might exert geroprotective effects in other ways.
Rapamycin and acarbose only took the 5th and 6th places, despite producing better results in mouse studies. Rapamycin is one of the most potent lifespan-extending drugs in animal models, and combining it with acarbose produces a synergistic effect. However, human mortality data is scarce.
The Longevity Shark Tank
Another unusual format test-driven at ARDD was the startup pitch. A string of startup founders came up before a panel of three actual investors to tell them about their ideas. We don’t know if the investors were convinced to actually invest, but a winner was proclaimed, and it was Aptah Bio, founded by Rafael M. Bottos around a big idea: solving the problem of RNA integrity.
Transcription from DNA to RNA is regulated by intricate and delicate machinery, which, unsurprisingly, gets dysregulated with age. Faulty transcripts produce faulty proteins – or none at all. Loss of RNA integrity over time has been linked to numerous pathologies and hallmarks of aging.
The longer the gene, the likelier its transcript is to suffer premature cleavage and splicing errors. According to Bottos, about 60% of human tissues demonstrate gene-length-dependent transcriptional decline, with the strongest effects in neural tissue.
Aptah’s candidate drug targets the protein complex U1-snRNP, “the RNA guardian” that ensures transcription quality. Abnormalities in U1 cause pathological RNA processing.
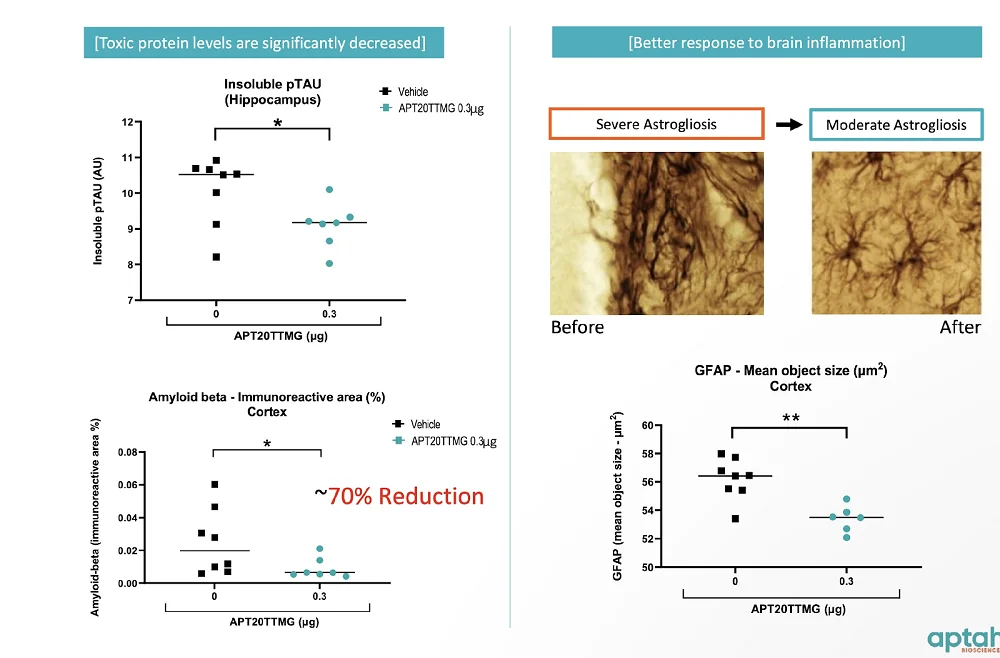
Aptah has conducted several in vitro and in vivo studies in which their candidate simultaneously reduced the expression of multiple toxic proteins, such as insoluble phosphorylated tau and amyloid beta, two well-known hallmarks of Alzheimer’s disease. The drug also showed promising results in a glioblastoma model.
Aptah has engaged in high-profile collaborations with UC San Diego and NASA. Brain organoids treated with Aptah’s compound will be launched into space later this year to study the possibility of rescuing accelerated brain aging in astronauts.
Eyes on the XPRIZE!
XPRIZE Healthspan, the biggest XPRIZE in history (101 million dollars) sent a whole team to ARDD. In a series of talks, its representatives explained the intricacies of the humongous prize and gave tips on how to apply. Jamie Justice, XPRIZE Healthspan executive director, introduced the endeavor, starting with the famed three-decades-long history of the XPRIZE.
The prize was years in the making, in part, due to the difficulty of defining endpoints. Since humans are so long-lived, demonstrating life extension by a novel therapy using mortality is problematic. After consulting world-renowned experts such as David Sinclair and George Church, the team settled on a different criterion: reversal of loss of function. If your therapy can demonstrate true reversal of age-related loss of function, such as muscle or brain function, you have a chance to win.
XPRIZE Healthspan was made possible by the relentless work and generosity of several people, starting with Peter Diamandis, XPRIZE founder. Read our recent interview with him for more details. Big contributions were made by Chip Wilson, founder of the fashion brand Lululemon, entrepreneurs Christian Angermayer and Sergey Young, Hevolution Foundation, and others.
Restoring epigenetic information
Harvard professor David Sinclair is probably the most recognizable face in the longevity field. As usual, the celebrity geroscientist and the best-selling author of Lifespan: Why We Age and Why We Don’t Have To gave a fast-paced talk and was ushered away immediately after.
During the minutes he spent on stage, Sinclair gave an update on his information theory of aging. This theory centralizes epigenetic changes, which are caused by the imperfect character of the DNA double-strand break response. This response is facilitated by the proteins SIRT1 and SIRT6, which also have the responsibility of keeping chromatin from unwinding, thus preventing unwanted gene expression. Basically, those proteins were given two jobs by evolution, and neither is being done flawlessly, which probably sounds familiar.
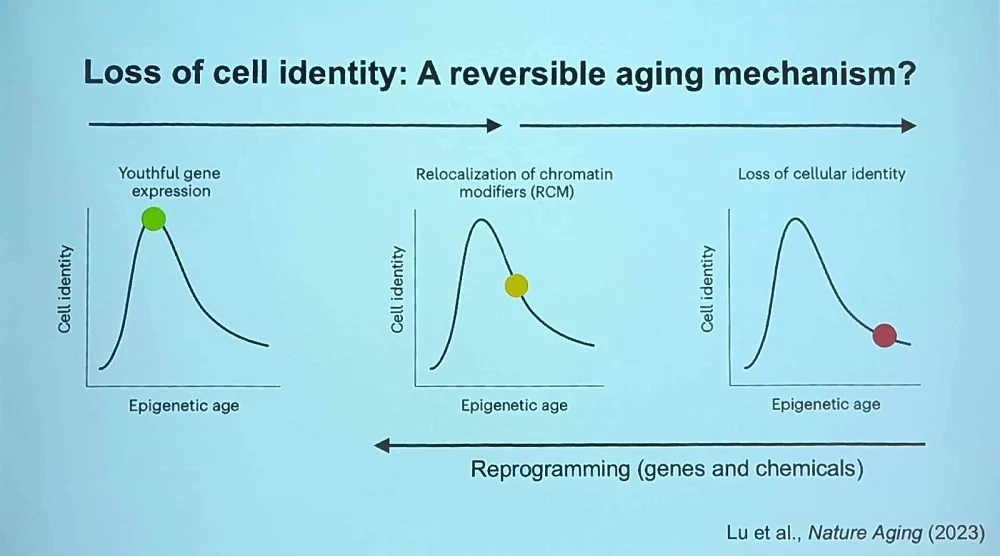
This arrangement gives us several decades of life, which means it’s good enough from the evolutionary standpoint. However, it causes the accumulation of epigenetic mutations (i.e., loss of epigenetic information, hence the theory’s name) and various age-related pathologies. According to Sinclair, cellular reprogramming suggests the existence of a backup copy of this information inside each cell, and his team is looking for this mechanism.
While we don’t know how it works yet, we can already apply it towards curing age-related diseases. Sinclair’s group has done groundbreaking research, restoring vision in animal models, including non-human primates, by reprogramming retinal ganglion cells (RGCs) in vivo.
The team uses three reprogramming factors out of the original Yamanaka’s OSKM cocktail, delivered using adenovirus-associated viral vectors (AAV2-OSK) in an inducible expression system for reliable and safe partial reprogramming. Sinclair’s company Life Biosciences, which also presented at the conference, is gearing up for human trials of this technology and might well become the first company to bring cellular reprogramming to clinic.
Sinclair talked about other possible use cases his group has been studying. Apparently, OSK treatment partially restores senescent cells’ transcriptome, suggesting it can work as a senomorphic. Peculiarly, cancerous cells treated with OSK change their morphology towards their original cell type and become much less active. “When cancer cells wake up to their identity, they freak out and kill themselves,” Sinclair said.
Sinclair’s group has also been successfully experimenting with in vitro reprogramming using small molecules. This treatment leads to “an almost complete restoration of nuclear integrity, even better than gene therapy,” he said, and can restore identity in cells that lost it many years ago.
Organ-specific aging
Another Harvard professor, Vadim Gladyshev, is fascinated with what aging is and how to measure it. Aging, he said in his talk, can be quantified on many levels – damage, functional decline, disease, mortality, and so on. Many epigenetic clocks are trained to predict mortality, and an important advantage of such clocks, according to Vadim, is that they can also predict the effects of interventions.
It is also important to distinguish between organismal and organ-specific aging. In different people, different organs age at different rates. Your heart can be younger than your liver and vice versa. Transcriptomic clocks are well-suited to capture organ-specific aging, since transcriptome can be decomposed into modules, and clocks can be developed from those modules (for example, a clock for the extracellular matrix).
Even organ-specific clocks can be trained on mortality data, which allows you to predict mortality of the organism from the age of an organ. Organ-specific plasma protein models can track organ-specific diseases, Vadim said. For instance, lung-specific models can predict chronic obstructive pulmonary disease (COPD), while liver-specific models can predict liver damage.
This approach yields many interesting insights. Associations of various factors with organ-specific aging can be measured. Smoking, unsurprisingly, is associated with increased aging in all organs. Alcohol consumption, on the other hand, produced mixed results: while it’s obviously bad for kidneys and the intestine, it’s good for the lungs and arteries. “If your arteries age fast, go to Bar 7,” Vadim joked, referring to the nearby establishment where the attendees enjoyed free booze every evening of the conference.
Jokes aside, it’s a serious matter. Vadim hopes that measuring organ-specific age will give us better diagnostics and more personalized interventions.

Bonus – part of the bespoke cocktail menu from Bar 7:

Maximum lifespan vs chronological age
In the last talk of the conference, another star geroscientist, Steve Horvath, currently with Altos Labs, continued with the theme.
While methylation clocks have been at the center of Steve’s scientific career, he also recognizes their limitations, such as a lack of biological interpretability: we don’t really know what mechanisms move the needle. Why is methylation such a strong predictor of morbidity?
Still, methylation clocks have a lot of appeal. Steve talked about “intuitive appeal” (if the molecule that carries the genetic information also encodes time, it suggests a profound relationship with aging), and “technical appeal” (methylation clocks are ready for human clinical trials, can be used in vitro, and can be multi-species).
Creating multi-species clocks has fascinated Steve for several years now. Pan-mammalian clocks are important for developing interventions, he said, because “whatever moves them, probably will work in humans.” Speaking further about interventions, Steve referenced Alex Zhavoronkov’s talk about deducing therapeutic targets from methylation data, calling it “the best talk in the longevity field ever.”
One interesting direction is clocks that predict the maximum lifespan of a species. It’s not as easy as it seems, as “biological processes that relate to time-to-death (of an individual) often differ from those that relate to maximum lifespan of the species,” Steve said.
As evidence to that, methylation sites used in epigenetic clocks (CpG sites) associated with maximum lifespan “show minimal overlap with those linked to chronological age”, and predicted maximum lifespan does not relate to human mortality risk in epidemiological cohorts.
Methylation signatures of long-lived species differ from those of short-lived species, and the rate of change of methylation seems to be a good predictor of maximum lifespan, but only in certain DNA regions (e.g., bivalent promoter regions). By definition, Steve said, for any biomarker that increases with age, its rate of change will be inversely correlated with the species’ maximal lifespan.








