How Superior DNA Repair Gives Bowhead Whales Longevity
- We might be able to make use of their unique protein.

Scientists have found a possible explanation for bowhead whales’ exceptional lifespan, and it might be translatable to humans [1].
More cells, less cancer
With some exceptions, body size is strongly correlated with longevity across species. While this can be explained evolutionarily (larger species have fewer extrinsic threats, which favors slow reproduction and longer lifespan), it presents a biological conundrum since larger animals consist of more cells. Cancer develops from a single cell that undergoes a series of oncogenic mutations. Hence, species with more cells should be more vulnerable to cancer. However, in nature, the opposite is usually true. This is known as Peto’s Paradox [2].
No magic is involved, of course. Larger animals simply developed more effective anti-cancer defenses. A few years ago, scientists found that elephants possess extra copies of the gene that produces p53, a key protein in cells’ programmed death (apoptosis) [3]. Studying these mechanisms may help find effective cancer cures for humans.
Think big, very big
This new study, published as a preprint on Biorxiv and authored by such prominent geroscientists as Vera Gorbunova, Vadim Gladyshev, and Andrey Seluanov, focuses on an even larger species: the bowhead whale (Balaena mysticetus). This phenomenal creature is the second-largest in the world, as it weighs up to 80 tons, and is thought to have a lifespan north of 200 years. The Inuit, who have hunted bowhead whales for centuries, say that these animals “live two human lifetimes”.
A cell must undergo more than one oncogenic mutation, or “hit”, to become cancerous. One of the reasons why humans are less likely to get cancer than mice is that in mice, two hits are enough, but to make a human cell cancerous, five are required [4].
However, the researchers found that bowhead whale fibroblasts require fewer hits than human fibroblasts to become cancerous. Whale cells also had the lowest p53 activity among all the species tested, which included mice and cows.
A protein that leads to fewer mutations
What if the answer lies upstream? Mutations often result from DNA breaks that can be caused by factors such as radiation and oxidative stress. Those breaks can be repaired, and just like other defenses, DNA repair mechanisms are better in some species than in others.
The researchers found that bowhead whales experienced much fewer mutations, indicating better DNA repair. Double-strand DNA breaks are addressed by two different mechanisms: homologous recombination (HR) and non-homologous end joining (NHEJ). The first one requires the presence of a homologous chromosome, which could only happen in a dividing cell. In post-mitotic (non-dividing) cells, NHEJ is the prevalent mechanism, and this is the one the researchers focused on.
NHEJ is a useful tool, but it is prone to mistakes that result in mutations. The researchers found that in bowhead whales, NHEJ is remarkably more efficient than in three other species and much more often results in faithful repair. Interestingly, in whales, the process was heavily skewed towards a particular type of error: single base pair insertions. Whale NHEJ also produced dramatically fewer large deletions, which are likely to be especially deleterious to a gene’s function.
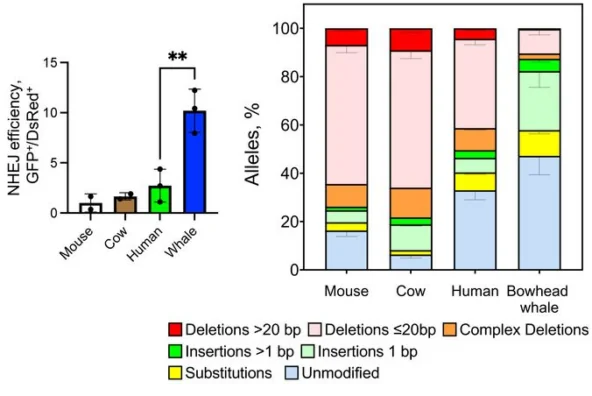
Among various proteins involved in NHEJ, one was remarkably abundant in whale fibroblasts: cold-inducible RNA-binding protein (CIRBP). Despite its name, it is induced not only by cold, but by some other types of stress as well, including hypoxia and ultraviolet radiation.
Works in human cells
Overexpressing the bowhead whale version of CIRBP (but not the human version) improved NHEJ efficiency in human cells 1.6-fold. Interestingly, it also led to a twofold increase in homologous recombination efficiency. Exposing human cells to hypothermic conditions also increased their NHEJ efficiency twofold.
While cold exposure has been shown to improve health, it is probably impossible for us to boost our DNA repair to whale-like levels by taking cold plunges. However, it might be achieved by using whale CIRBP, which only slightly differs from its human counterpart.
The researchers note that such an upstream solution to the problem of oncogenic mutations is superior to both apoptosis and cellular senescence, since it does not require killing cells or creating dysfunctional cells, which, over a long time, might become deleterious to the organism, such as when killing off too many irreplaceable neurons.
Vera Gorbunova mentions this discovery in our recent interview.
Literature
[1] Firsanov, D., Zacher, M., Tian, X., Zhao, Y., George, J. C., Sformo, T. L., … & Gorbunova, V. (2023). DNA repair and anti-cancer mechanisms in the longest-living mammal: the bowhead whale. bioRxiv, 2023-05.
[2] Peto, R. (2016). Epidemiology, multistage models, and short-term mutagenicity tests. International Journal of Epidemiology, 45(3), 621-637.
[3] Abegglen, L. M., Caulin, A. F., Chan, A., Lee, K., Robinson, R., Campbell, M. S., … & Schiffman, J. D. (2015). Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. Jama, 314(17), 1850-1860.
[4] Rangarajan, A., Hong, S. J., Gifford, A., & Weinberg, R. A. (2004). Species-and cell type-specific requirements for cellular transformation. Cancer cell, 6(2), 171-183.








