How to Defeat Aging? Two Scientists Offer Their Visions
- The argument is about what parts of aging are feasible to reverse.

In a much-anticipated debate, prominent aging researchers Aubrey de Grey and Peter Fedichev presented their competing, but also overlapping, theories.
Gentlemen, draw your laser pointers!
When the non-profits Foresight Institute, Open Longevity, and Say Forever had the idea to hold debates on the best strategy to defeat aging, there was little question about whom they should invite first. Aubrey de Grey, head of LEV Foundation and one of the faces of the longevity field, and Peter Fedichev, CEO of Gero and a rising star in the same field, already had an impromptu debate last year in Zuzalu, the longevity/crypto/AI-themed pop-up city in Montenegro. I had the honor to witness that clash of titans, which kept a small but dedicated crowd on its toes for more than two hours.

The impromptu debate in Zuzalu. Photo: Arkadi Mazin
Now, almost exactly a year later, the two scientists agreed to settle the score, with a panel of five esteemed judges and a $10,000 prize at stake. The debate mostly clarified the participants’ positions, which turned out to share many similarities but also some important differences. What is undebatable, though, is that this was one of the most fascinating and eye-opening longevity-related events of the year and that such debates should become a frequently honored tradition.
The jury included Prof. David Furman (Buck/Stanford), Prof. Dorota Skowronska-Krawczyk (UCI), Prof. Guo Huang (UCSF), Prof. Thomas Stoeger (Northwestern), and Prof. Mattew Yosefzadeh (Columbia).
At the center of the disagreement was our ability to reverse aging in the near future. Aubrey is much more optimistic about that than Peter. From that rather straightforward point, the journey down the rabbit hole began.
The importance of education
Aubrey took the stage first, focusing this initial part of his talk on the current longevity ecosystem rather than the science of aging reversal. The first thing we can do to extend the human lifespan, he said, is education. In this context, he mentioned several organizations, including Longevity Biotech Fellowship, which educates people seeking a career in longevity, and Lifespan.io, which he called “a fantastic, really important organization”.
Warm words were also reserved for the XPRIZE in healthspan, the Alliance for Longevity Initiatives, and the Dublin Longevity Declaration – “the single most important piece of advocacy in several years.” Aubrey praised “the emergence of new jurisdictions,” first and foremost, Prospera, the special economic zone in Honduras, which offers a streamlined and efficient regulatory system for young biotech companies.
The reign of entropy
When his turn arrived, Peter dived into the biology part head-first. His theory, developed in a series of publications starting from 2015, borrows heavily from his background in physics. Peter claims that he and his company were able to detect two types of age-related signals in some biological data, including epigenetics.
When they performed a principal component analysis on their methylation dataset, Peter said, they saw one principal component increasing linearly with age and the other one increasing exponentially. Here is how Peter interprets this: normal cellular processes produce heat, which, in turn, inevitably causes some amount of damage, such as genetic and epigenetic alterations. This damage is stochastic, and the alterations are unrelated to each other. As a result, all cells accumulate different patterns of those changes.
Some of those changes are benign, yet, with time, they begin to affect cellular function, creating stress. The body reacts to this stress by activating various compensatory mechanisms, but since everything in the organism is tied together so tightly, problems in one place create more problems in another, and the organism gets more and more out of balance.
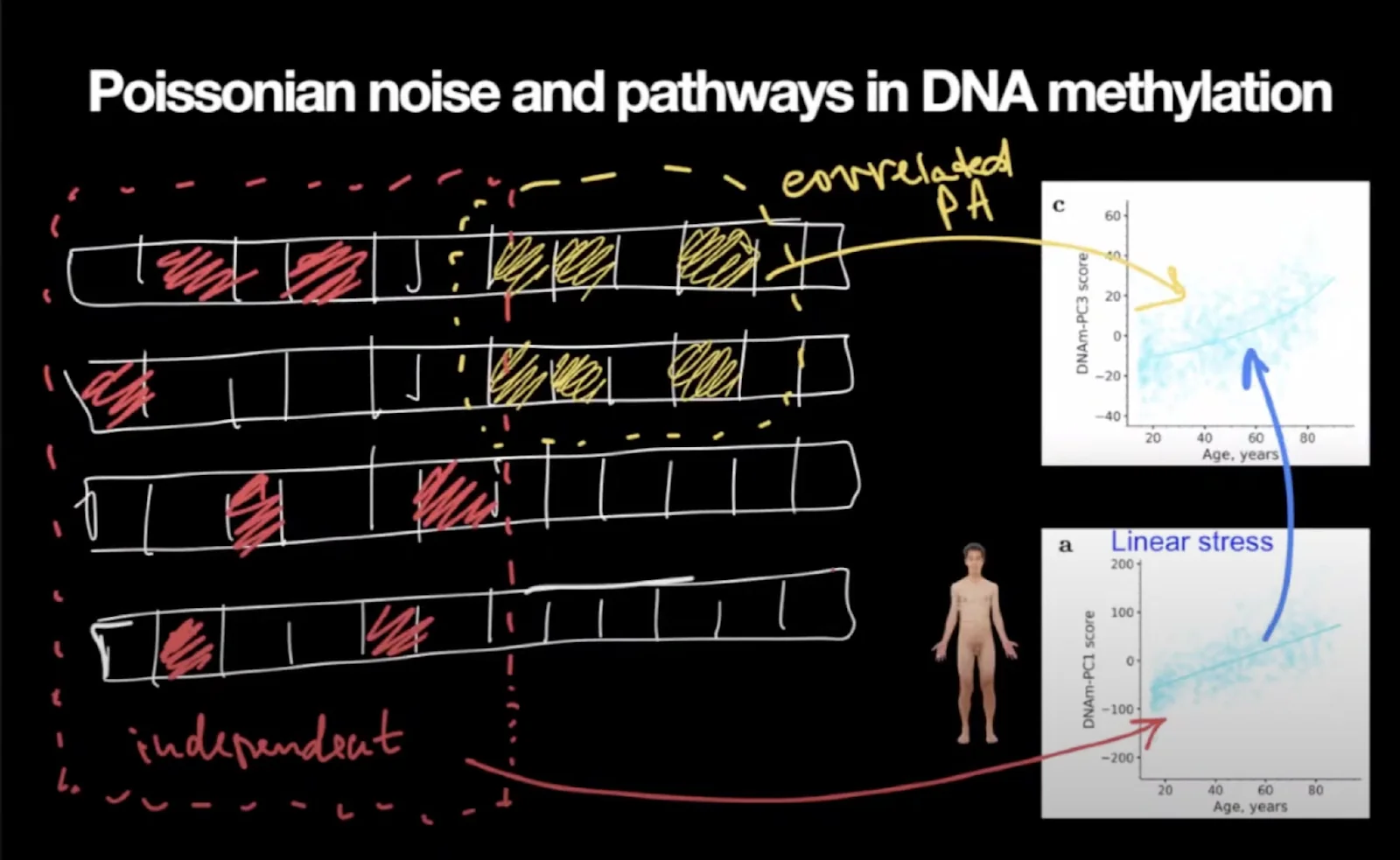
Yellow squares are methylation changes that reflect the cell’s normal workings, i.e., biological pathways performing essential functions. Pathways correlate with each other. This work produces heat which produces stochastic damage (red squares). The linear accumulation of this damage (the bottom graph) induces even more stress responses, which is reflected by the top graph’s increasing curvature.
Humans are already an exceptionally long-lived species, which means we have excellent mechanisms of controlling damage and stress (not as good as some other species, such as the naked mole rat or the bowhead whale). Therefore, in humans, the accumulation of instability occurs very slowly, but it occurs nevertheless, and in the last part of our lives, it becomes conspicuous, with numerous things going out of control in a feedback loop, causing pathology. As more stress accumulates, the organism becomes less resilient to it and eventually “goes over the cliff”, leading to death.
As an illustration (conceived by me, not Peter), imagine a tightrope walker. He is very good at what he does, so, initially, he walks nonchalantly on a brand-new, perfectly tight rope over the abyss, experiencing little discomfort. Imagine now that the rope starts disintegrating slowly due to the elements. At first, the walker manages to expertly correct his stance when he encounters those slight imperfections, but as they multiply, it’s becoming increasingly hard, and he’s spending more and more energy, even as he’s getting increasingly tired.
At this point, even a small increase in the density of imperfections causes a large increase in energy expenditure. We see that he’s walking slowly, noticeably wobbling. Finally, one more step becomes too much – he starts waving his hands wildly in a desperate attempt to keep his balance, which only gets him more and more out of balance. I don’t need to tell you what happens next.
In this model, it is not the biomarkers that increase exponentially. In fact, probably not a single biomarker changes exponentially with age; otherwise, we’d see very large increases in older people, and we only see something like that in life-threatening medical situations.
Instead, what we usually see is a linear increase in the variability and/or a change in the average level of the biomarker. Take blood pressure for example: with age, it increases gradually, and its variability increases as well. Both changes are considered alarming. Levels of cholesterol generally slowly increase with age, and levels of VO2max decline.
So, the exponential signal that Peter is talking about is a composite measure of instability. We might not be able to map it to all the specific interdependent molecular processes it reflects, but according to Peter, we see that it strongly correlates with morbidity and mortality. Without knowing what it is exactly, Peter calls it “the dynamic component of aging.” The second one, which increases linearly due to the stochastic damage caused by metabolic processes, reflects the increase in entropy and is named “the entropic component of aging.”
An important part of Peter’s theory says that various species age differently. Mice live for 2-3 years because they are, essentially, not very good at walking this tightrope. Or, as Peter puts it, mice are “tiny explosions”: they start disintegrating almost immediately after they are born, entering the death spiral that humans enter many decades later. This makes mice popular models in biomedical research in general, although many interventions that work in mice fail in humans, but especially poor models in aging research.
Aging in mice is dominated by the dynamic component, which also reacts well to interventions because it reflects interdependent biological pathways. Affecting a pathway may bring back some of the stability and calm things down overall, and this, Peter says, is what we see with our current geroprotectors.
However, in humans, the dynamic component becomes dominant (“we become mice”, as Peter puts it) only during the last couple of decades of life or so. By affecting this component with interventions without stopping the entropic (stochastic) damage from accumulating, we can add, Peter estimates, about a decade to average life expectancy. If we can also stop the entropic damage in its tracks, we can keep a person stable much longer.
The central tenet of Peter’s theory is that reversing the entropic element of aging is extremely hard, because entropy doesn’t like to be reversed (try unmixing two liquids or, if you’re up to a really hard challenge, unbreaking an egg). Without reversing it, he argues, we cannot achieve actual rejuvenation. This bleak prospect, however, is only for the foreseeable future since, technically, Peter admits, there is no “hard limit” on reversing entropic damage, and with technology much more advanced than ours, this can be done.
So, Peter’s answer to the debate’s main question is that we need to concentrate on slowing the accumulation of entropic damage. Otherwise, according to this view, we can only achieve very limited life extension. If we manage to stop the entropic element and reign in the dynamic element of aging, we can keep people at their current age for a long time. However, we cannot rejuvenate people without finding a way to reverse the entropic element, which is extremely hard.
The maintenance approach
Next, Aubrey took the stand to paint his picture of aging.
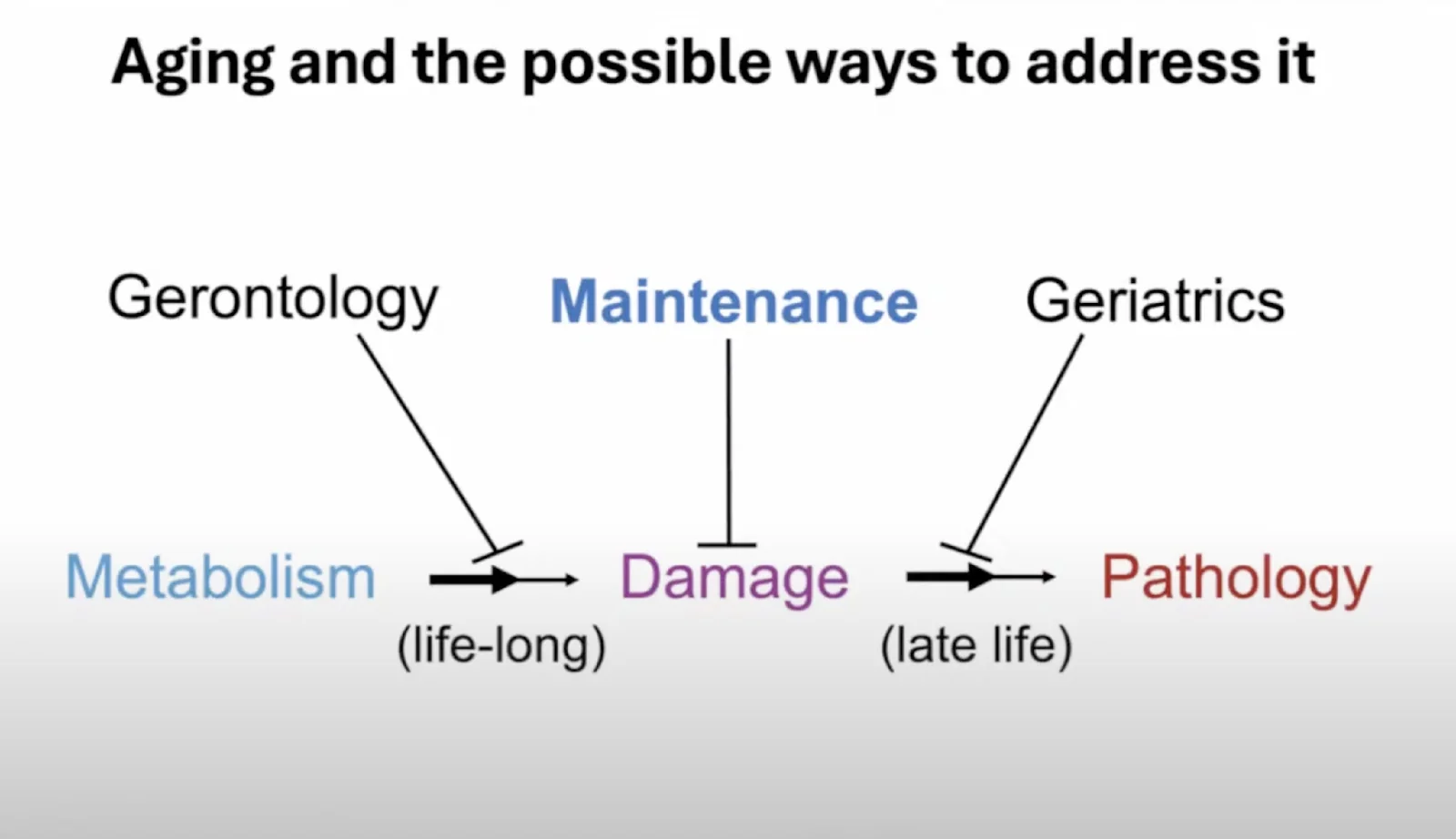
Aging, he said, consists of the combination of two processes. The first one is “a lifelong process whereby metabolism, in other words, the network of processes that keeps us alive from one day to the next, generates changes to the molecular and cellular structure of the body that accumulate over time.” This is what Aubrey calls damage. Accumulation of damage due to normal metabolic processes looks a lot like Peter’s “entropic element of aging.” With time, the increasing damage causes pathology.
Aubrey offered an interesting narrative of how medicine’s efforts to counter aging have changed over time. Encouraged by early successes against infectious diseases, scientists thought it would be just as easy to reverse aging-related pathologies. Obviously, it did not happen. Attempts to alter metabolism so that it creates less damage have not been very successful either. This is why Aubrey has always seen removing damage (“the maintenance approach”) as the only feasible way to disconnect metabolism from pathology and, hence, to slow and hopefully reverse aging.
Since metabolic processes cause many types of damage, a key part of this approach is combination therapies. Unfortunately, the whole system of incentives, both in academia and in biotech, is built to favor single interventions. Aubrey himself is working on the combination approach in the ongoing Robust Mouse Rejuvenation (RMR) study. Still, combining two, three, or even four therapies is just the first step.
An important feature of Aubrey’s concept is that developing pathologies heavily influence the amount of damage produced by metabolism. Again, this bears some similarity to Peter’s idea that pathways produce increasingly more stochastic damage.
 Aubrey said that, like Peter, he defines two types of damage: chemically defined damage and chemically undefined damage. The first type is, for example, “something that can be distinguished as damage versus not damage by an enzyme.”
Aubrey said that, like Peter, he defines two types of damage: chemically defined damage and chemically undefined damage. The first type is, for example, “something that can be distinguished as damage versus not damage by an enzyme.”
This includes the accumulation of waste products, such as amyloids and crosslinks (chemical bonds between proteins in the extracellular matrix) along with the loss of cells, including stem cells. On the other hand, genetic and epigenetic changes are “chemically undetectable”: enzymes cannot identify sequences with deleterious alterations.
According to Aubrey, both he and Peter agree that it is possible, even if not easy, to remove damage of the first type, but they disagree on how much harder it is to fix the damage that Peter calls entropic and Aubrey calls undetectable or informatic. Notably, although some elements of Aubrey’s and Peter’s theories resemble each other, they don’t necessarily overlap perfectly. Comparing the two theories will probably take more work.
“We know that (informatic damage) can be removed,” Aubrey said, “if you’ve got some kind of oracle, an external source of information that says, this is damage, this is not damage. But we don’t have that oracle, and we certainly don’t have a way to communicate that oracle to cells.”
However, Aubrey is optimistic because of “informatic redundancy” – the idea that the information about a particular cell type’s “pristine” epigenome is retained even if a certain amount of epigenetic damage accumulates. Partial cellular reprogramming is a way to restore this information without sacrificing the cell’s differentiation and without the need for an oracle.
Aubrey is still not sure that experiments in partial reprogramming tell us this, but he thinks that recent research leans towards his hypothesis. His disagreement with Peter is that “the amount of rejuvenation that we can do without an oracle is much greater than it might seem.”
David Sinclair uses an appropriate metaphor. Sinclair, too, puts a lot of hope in restoring epigenetic information using partial cellular reprogramming. He has also achieved some practical success, restoring crushed optical nerves in rodents and non-human primates.
Sinclair likens partial reprogramming to removing scratches from an old CD (hopefully, you remember what that is). Scratches, i.e., epigenetic noise, obscure valuable epigenetic information, but the latter can still be restored, at least up to a certain point. Sinclair also suspects that there is a “backup copy” of this epigenetic information hidden in the cell. He does not have a sound theory about the mechanism yet, but in one of his recent papers, he makes interesting suggestions about how a cellular mechanism of recording epigenetic changes might work.
“The epigenome is what matters”
In the second round, Peter agreed with Aubrey on two issues. First, that a strong positive feedback loop between pathology and the amount of damage produced by biological processes exists, and it kicks in much faster in mice, which shapes the way they age. Second, he agrees about the existence of defined and undefined damage.
“I think it’s a very good definition,” he said, “because we are an evolutionarily sophisticated species, meaning that most of the time, we have options to remove a lot of defined damage. We may not use them for whatever reasons, but the options exist.” Hence, by ramping up those inbuilt mechanisms, we can enzymatically remove a lot of this chemically defined damage.
Still, Peter brought up other types of undefined damage, such as activated retrotransposons that insert themselves back into the genome – damage that, he said, will be much harder to remove. He also mentioned genetic mutations, the rate of which is strongly associated with lifespan across species. Our ability to identify and fix those types of damage might be limited. “There is no evidence,” Peter said, “that (epigenetic mutations are) even the largest part of the story.”
Aubrey countered by noting that cellular reprogramming “just works,” and since differentiation affects only the epigenome but not the genome, “it must be telling us that the epigenome is what matters.” He agreed with Peter that partial cellular reprogramming has not shown a drastic effect on lifespan in mice and that there seems to be a narrow therapeutic window (applying too much of the reprogramming factors kills the animal).
However, he noted, all the experiments we have seen only tested cellular reprogramming alone, without fixing other types of damage. This might be the reason why effects from reprogramming max out quickly. Another reason is that we are just not very skilled at it yet. In the next round of RMR, Aubrey plans to include partial reprogramming so that it can be tested in combination with other interventions.
Next, Aubrey asked Peter about some of the interventions to go after genetic and epigenetic damage. Peter suggested that it might be possible to remove most damaged cells, such as senescent or cancerous cells. Peter sees studying biological noise (the inevitable stochastic damage stemming from the operation of the biological machine that is our body) as essential and is glad to see this field of research growing quickly.
Hundreds of years or a couple of decades?
At this point, a judge’s question returned the discussion to its original topic: Can we rejuvenate the human body? Aubrey answered first by saying that we can definitely “restore some aspects of the structure, function, and composition of the body,” which is rejuvenation by definition. The real question, however, is can we achieve comprehensive rejuvenation, or are there changes to the body’s structure and composition that cannot be reversed with foreseeable technology?
“My belief,” he said, “is that the amount of informatic redundancy that exists in the genome and the epigenome is sufficient for us to be able to comprehensively rejuvenate the body in the foreseeable future.” Aubrey added that he always thought that fixing chemically identifiable damage can only get us so far, and at some point, the accumulation of mutations and epimutations in itself will become deadly. However, he is convinced that the relative contribution of those mutations to aging is so small that, if we find ways to remove all other damage, we will be able to live for hundreds of years.
“Peter’s contention, as I understand it,” he continued, “is that this limit is going to hit us within as little as 10 or 20 years beyond where we can already get. In other words, we can only get 10 or 20 years from the chemically detectable aspects of rejuvenation. I believe, actually, no, we can probably get hundreds of years.”
Peter generally agreed with Aubrey’s representation of his position. He thinks that the longevity field and the pharma industry are mostly going after chemically identifiable damage, and we can only expect limited gains from this approach. The only way to stop aging is by preventing the growth of entropy, which is quite hard (“to struggle against the second law is the last thing that you want to do”) – and that’s even before we start thinking about reversing entropy, which would be required for true rejuvenation.
We go to the scorecards
After another couple of questions from the judges, the debate was officially scored 38 for Aubrey, 42 for Peter, and the proceedings moved on to questions from the audience, which turned out to be just as challenging and insightful.
One of the questions involved an embryonic reset: the apparent erasure of damage, except for genetic mutations, that occurs somewhere during early embryonic development and allows old organisms to produce perfectly young offspring in the never-ending cycle of life. Can an embryonic reset be used to create rejuvenation therapies?
Both Aubrey and Peter responded to the effect of an embryonic reset being analogous to the factory reset of a cell phone, which makes it hard to use it for therapies in vivo. However, the mechanisms of such an embryonic reset and its relation to the Yamanaka factors are being actively studied, including by Vadim Gladyshev’s team at Harvard.
Another question pointed at Peter was whether we have proof of a causative relationship between entropic damage and aging. Peter admitted that, at the moment, it was “a neat theoretical argument that relies on a certain physical intuition” and the most natural explanation.
“I have to confess,” he said, “that direct evidence does not exist because we don’t have an experiment where this damage is either reduced or stopped, and then we would see some effect on lifespan. Mice simply don’t give us such an opportunity right now, so at this time, this argument flows from modeling. We have a model that relates this linear damage to this hyperbolic activation of stress responses that actually kill the animal.”
A member of the audience pointed out that entropy can be reversed if you pay an energetic penalty. In other words, entropy can be reversed in an open system that can draw energy from outside.
Peter said that the problem was not the amount of energy needed – according to estimates, it would only take one cookie a day to fix all our DNA – but the amount of information we have about genetic and epigenetic states and the level of control: “If you can measure all states in your system, and if you can come up with a very exact intervention, you can do whatever you want.”
An elephant in the room addressed in another question was tissue and organ replacement. This direction holds a promise of major shortcuts in our quest for body rejuvenation. After all, do we need to care about the accumulation of damage on a cellular level when we can replace large chunks of our body with perfectly young ones?
Aubrey said that we should invest in this direction but not make it our main focus, although “we’re quite likely to end up needing to do macroscopic interventions for a short while as we reach longevity escape velocity.” Peter’s take was more sympathetic: he called organ replacement “the last resort against the second law of thermodynamics,” which has a lot of potential.
Hope is the last thing ever lost
With that, the debate came to a close, leaving the audience wanting more. It was admirably amicable and thoughtful and showcased the deep mutual respect between the debaters. Among the noteworthy things that I felt did not get proper attention are two promising directions of research, both of which are related to the nefarious entropic damage at the center of Peter’s theory.
First, many species have developed superior mechanisms of damage control, including DNA repair, and researchers such as Vera Gorbunova are working on understanding those mechanisms and translating them for humans. Second, there are budding attempts to reverse the burden of mutations. At the recent Rejuvenation Startup Summit in Berlin, the company Matter Bio presented its vision for identifying and fixing DNA mutations in vivo.
For me, the main takeaway from the debate was more in line with Aubrey’s optimistic vision. Both slowing and reversing aging are theoretically possible, and there are numerous avenues we can pursue toward both of those goals (just take a look at our Rejuvenation Roadmap).
Eventually, we will probably end up with a wide range of interventions, which would include effective waste clearance, cellular reprogramming in vitro and in vivo, clearance of aberrant cells and mitochondria, restoring the extracellular matrix, tissue and organ replacement, elimination of mutations, and many others. While this approach does not look like a “silver bullet” for aging, it gives us realistic hope.








