Identifying Mitonuclear Genes for Longevity
- Nuclear genes that affect the mitochondria may have a significant effect on lifespan.

Publishing in GeroScience, a team of researchers that included Nir Barzilai and Matt Kaeberlein examined genes that may affect both mitochondria and lifespan [1].
From the mitochondria to the nucleus
Over time, evolution has moved mitochondrial DNA from the individual mitochondria to the nucleus, where they are better protected. SENS Research Foundation, in conjunction with Lifespan.io, has conducted research into moving more of these genes there.
However, the mitochondria-affecting genes that are already in the nucleus (mitonuclear genes) are also worthy of examination. Mitochondrial dysfunction is a hallmark of aging, and these researchers found it likely that gene variants in this area may have effects on lifespan. To that end, they examined a well-established cohort of approximately 660 mitonuclear genes in 496 centenarians and 572 controls, all of whom were of Ashkenazi Jewish descent.
A new push of research
The researchers begin their paper by discussing mitochondrial dysfunction and its roots before moving on to centenarians: people who have lived for at least 100 years. Extensive previous research has shown that there is a significant genetic component to this kind of longevity [2], and these researchers have previously enumerated several genes that affect it through signaling pathways [3]. This mitonuclear research builds upon that previous work.
To identify their candidate genes, the team fished through a substantial number of rich databases, including the Human Ageing Genomics Resources and the Digital Ageing Atlas, and the functions of these genes was categorized through other databases, such as Reactome and the Gene Ontology Resource. Variants of the potential genes were heavily analyzed: one algorithm was used to predict whether mutations would affect the resulting proteins, and another system determined how mitochondrially localized the genes were.
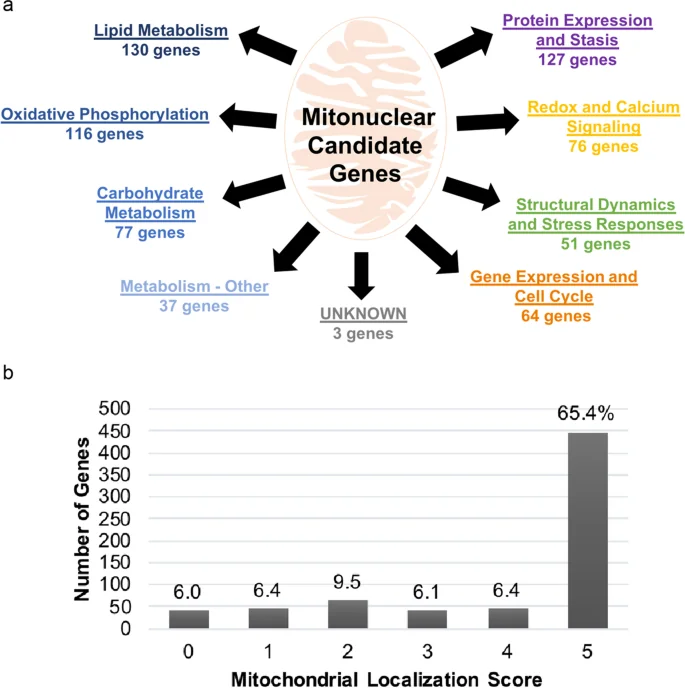
The researchers report that about a hundred of the mitonuclear genes have variants that are associated with age-related diseases, mostly related to metabolism and the immune system. Only six candidates were found to be directly correlated with lifespan, and the researchers found only one gene variant, rs689454, that met their threshold of multiple testing correction significance, which drives the p value to a very low number. This is a variant of NQO1, a gene responsible for preventing cyclic molecules from forming free radicals.
The researchers found a total of 76 genes that they deemed likely to affect lifespan, even though they could not prove this to the multiple testing standard. These genes were responsible for such things as ketone metabolism, mitochondrial morphology, and roles in the metabolism of cholesterol and fatty acids.
The researchers also focused strongly on rare variants. They found particular interest in two extremely rare mutations, which have only been detected in centenarians, of the gene coding for the LRPPRC protein, whose crystal structure has never been analyzed and whose role in longevity has never been explained. The team believes that this protein is a good target for future research.
Conclusion
This is an extremely in-depth paper with a great amount of computational resources utilized and many interesting avenues earmarked for future analysis. However, many of its findings do not pass the desired statistical significance. It seems to be that the cohort of approximately a thousand people is simply too small to offer any certainty about the effects of truly rare gene variants.
While this paper advances the field, if we are to truly get anything useful out of this sort of analysis, we must understand in detail what these genes are and what biological effects their mutations are having – a task that is extremely difficult even for the researchers and computers of 2022.
Literature
[1] Gonzalez, B., Tare, A., Ryu, S., Johnson, S. C., Atzmon, G., Barzilai, N., … & Suh, Y. (2022). High-throughput sequencing analysis of nuclear-encoded mitochondrial genes reveals a genetic signature of human longevity. GeroScience, 1-20.
[2] Schoenmaker, M., de Craen, A. J., de Meijer, P. H., Beekman, M., Blauw, G. J., Slagboom, P. E., & Westendorp, R. G. (2006). Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. European Journal of Human Genetics, 14(1), 79-84.
[3] Ryu, S., Han, J., Norden‐Krichmar, T. M., Zhang, Q., Lee, S., Zhang, Z., … & Suh, Y. (2021). Genetic signature of human longevity in PKC and NF‐κB signaling. Aging cell, 20(7), e13362.








