Inhibiting a Fundamental Factor in Brain Inflammation
- This approach tackles a major source of inflammaging.
Researchers have devised a method of reducing brain inflammation by creating a long-lasting inhibitor of the inflammatory factor NF-κB.
Targeting inflammaging at its roots
This study, published in the Nature journal Experimental & Molecular Medicine, begins with a discussion of age-related chronic inflammation (inflammaging) and its contributions to aging. Specifically, the researchers focus on neuroinflammation, which occurs when age-affected brain microglia begin sending out pro-inflammatory signals, particularly the cytokine NF-κB [1]. While considerable research has elucidated many of the fundamental reasons why this signaling occurs [2], treatments have remained elusive.
NF-κB, in particular, has been well documented; many papers on age-related diseases have pinpointed it as a problem and potential target [3]. However, while these researchers have noted that despite the existence of more than 700 NF-κB inhibitors in the laboratory, there is not a single one that has gone through the clinical trial process.
These researchers’ candidate is a variant of a known natural inhibitor, IκB. Replacing two of its amino acids prevents this protein from being degraded by cells as the natural version would be; this engineered super-repressor is termed srIκB, and it is intended to linger in the cellular cytoplasm and inhibit NF-κB in a long-lasting way.
As their delivery vector, the researchers have chosen exosomes, cellular messengers that do not stimulate the immune system [4], and the exosomes loaded with the super-repressor are called Exo-srIκB. In previous work, this research team has used Exo-srIκB to treat inflammatory diseases in animal models [5]; this, however, is their first foray into tackling brain inflammation.
Directly affecting aspects of inflammation
In the researchers’ first experiment, they examined the brains of 2- to 3-month-old mice and compared them with the brains of 21- to 22-month-old mice. As expected, the cytokines and inflammatory factors were significantly greater in the old mice, and the amount of natural IκB was lower. Leukocytes had infiltrated the brains of the old mice, and a gene expression analysis revealed a broad increase in inflammatory factor production.
The researchers then injected pairs of 2- to 3-month-old and 18- to 22-month-old mice with Exo-srIκB for three days, along with control groups receiving empty exosomes. The older mice given Exo-srIκB had considerably lower levels of key inflammatory factors, including interleukins such as IL-1α. They also had significantly lower levels of immune B cells and macrophages compared to their control group, meaning that the immune systems had reduced responses to inflammation. Genes relating to leukocyte migration and activation were downregulated as well.
Oligodendrocytes, play supportive roles in the functioning of the brain, such as myelination, and become more oriented towards inflammation with age. However, this age-related shift was largely reduced in the older mice given Exo-srIκB. Interestingly, however, the number of oligodendrocytes engaged in initial myelination was also reduced with the treatment; the researchers hypothesize that this is due to less need for it, as inflammation decreases myelination.
Astrocytes, which also play a supporting role in the brain, did not appear to change how they behaved. Concerningly, the numbers of some cells were changed in the same direction with Exo-srIκB as with aging. However, the endothelial cells appeared to move towards a more youthful phenotype, with brain permeability being decreased.
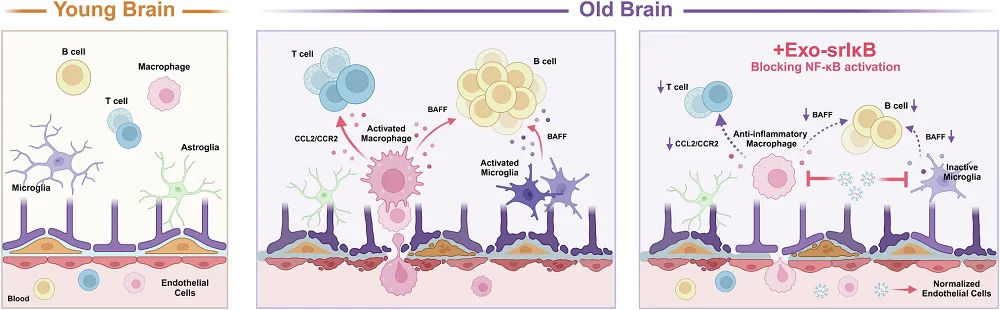
Intercellular communication was also significantly affected. Pathways involved in chemokine activation, which encourage B cells to infiltrate the brain, were significantly reduced in the old mice given Exo-srIκB. However, other pathways relating to T cells seemed to be even stronger than before, which, as these researchers discuss, may explain why the T cells continued to be prevalent even after Exo-srIκB treatment.
While it is clearly not a complete solution by itself, these researchers believe that “Exo-srIκB may serve as a potent therapeutic agent against pathological age-related inflammatory processes, especially those that target macrophages and microglia.” They note that while they used high concentrations of this protein, it did not appear to have any significant side effects. However, the populations used were low, and this was only a mouse study conducted over a limited time period. Further work will need to be done to determine if this approach could work in human beings.
Literature
[1] Rawji, K. S., Mishra, M. K., Michaels, N. J., Rivest, S., Stys, P. K., & Yong, V. W. (2016). Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain, 139(3), 653-661.
[2] Hammond, T. R., Dufort, C., Dissing-Olesen, L., Giera, S., Young, A., Wysoker, A., … & Stevens, B. (2019). Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity, 50(1), 253-271.
[3] Barnes, P. J., & Karin, M. (1997). Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. New England journal of medicine, 336(15), 1066-1071.
[4] Shiue, S. J., Rau, R. H., Shiue, H. S., Hung, Y. W., Li, Z. X., Yang, K. D., & Cheng, J. K. (2019). Mesenchymal stem cell exosomes as a cell-free therapy for nerve injury–induced pain in rats. Pain, 160(1), 210-223.
[5] Chae, J. S., Park, H., Ahn, S. H., Han, E. C., Lee, Y., Kim, Y. J., … & Kim, W. J. (2023). The effect of super-repressor IkB-Loaded Exosomes (Exo-srIκBs) in chronic post-ischemia pain (CPIP) models. Pharmaceutics, 15(2), 553.







