Magnitude Reveals a New Target for Aging Therapeutics
- This molecule increases autophagy in a well-known model of aging.

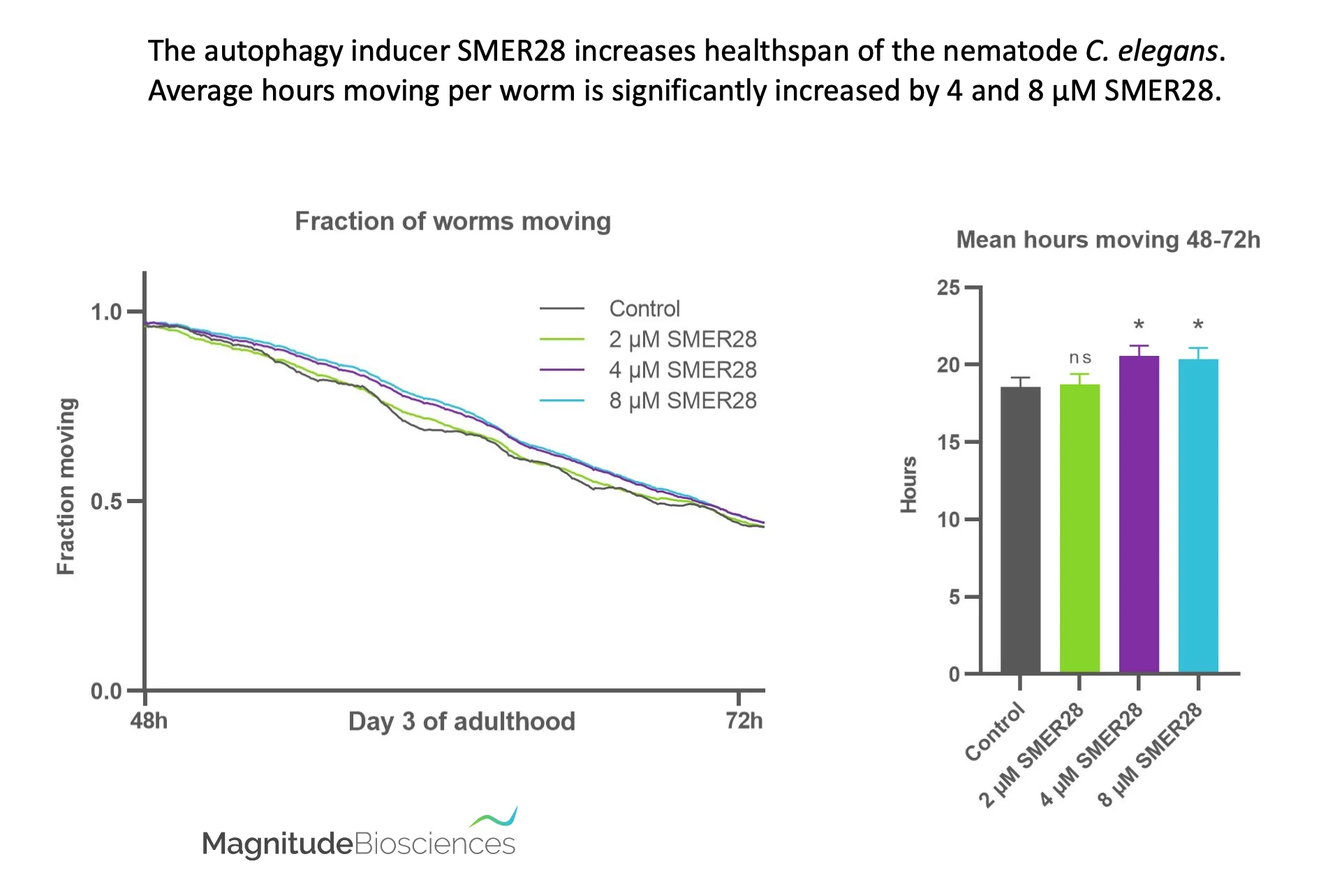
At Longevity Summit Dublin, Ethan Perlstein and Magnitude Biosciences revealed that a small molecule, SMER28, improves longevity in C. elegans, a common model of aging.
Background
One of the greatest challenges for biomedical sciences is how to slow ageing. Ageing is the major risk factor for almost all chronic diseases. And it has been estimated that one extra year of working life would increase UK GDP by 1%. Ethan Perlstein and others found that a small molecule called SMER28 was an inducer of autophagy. This earlier research used a multi-species approach to drug discovery that started with a yeast chemical modifier screen and was followed by studies in flies and human cells to further understand the mechanism of action. Subsequently, SMER28 was shown to improve models of anemia in human hematopoietic stem cells, zebrafish and mice. However, there was no known effect on Healthspan, and the target remained unknown.
Ethan identified the contract research organisation Magnitude Biosciences to investigate the efficacy of SMER28 to promote health and slow ageing, as he was familiar with how studies in the nematode C. elegans could be translatable for drug discovery, and that Magnitude Biosciences had unique capabilities and a reputation for strong data reproducibility as led by CEO David Weinkove.
Findings
Magnitude Biosciences revealed SMER28 showed toxicity at relatively low concentrations, but using their unique Automated Imaging Platform, they found a range of concentrations with a robust positive effect on Healthspan. Establishing a dose response of SMER28 also demonstrated the sensitivity of Magnitude’s technology to identify tight therapeutic windows of compound concentrations that slow ageing.
Perspective
Excitingly, the target of SMER28 was recently identified by Wrobel et al (2022) in human cells as VCP (p97). This target is well conserved in C. elegans. The homologue CDC-48.1 has 80% identity with the human protein, opening up an exciting opportunity to further study SMER28 in vivo to determine the mechanism for slowing ageing. The C. elegans cdc-48.1 mutant can be used to validate the target, determine if the toxicity remains in the absence of the target, and if so, ID any off-target effect. This work would provide a platform to discover new molecules that would be derivatives of SMER28 that hit the desired target to slow ageing but avoid the toxicity. This new safe molecule could be used to develop therapeutics to slow ageing in humans.
Press Contact: email: jess@magnitudebiosciences.com phone: +44 7566 236827, Ethan Perlstein and David Weinkove are available for interview.
About Magnitude Biosciences
Founded in 2018, Magnitude Biosciences is a specialist CRO, pioneering the use of the nematode C. elegans to help clients across industry and academia to propel their research projects via their unique automated imaging technology and team of multidisciplinary experts. They share a vision of bringing this unique approach to help accelerate the development of new drug therapies and other research advances to improve human and environmental health. Their studies are highly customised, with a focus on generating high-quality, reproducible data in key research areas including early preclinical drug discovery projects, ageing, microbiome, neurodegenerative diseases, environmental toxicity, reproductive toxicity, and C. elegans transgenics.
About Ethan Perlstein
Ethan Perlstein is a leading entrepreneur and CEO of Perlara, renowned for drug discovery for rare diseases in particular. Throughout his career, Ethan has gained a wealth of research experience in genetics, neuroscience, cell biology drug discovery and pharmacology and is very experienced with genetically amenable model organisms, such as yeast and the nematode C. elegans. In addition to his biomedical and biotech bench research, Ethan has led programmes to build pipelines of promising therapeutics by taking advantage of advances in the field to propel the discovery and development of life-changing interventions.
SMER28 target identification
Wrobel et al 2022. Compounds activating VCP D1 ATPase enhance both autophagic and proteasomal neurotoxic protein clearance. https://doi.org/10.1038/







