Making Genetically Engineered Stem Cells Viable
- Growing and selection are crucial.

Researchers publishing in Cell Stem Cell have announced a new method of accurately and rapidly cloning genetically engineered stem cells.
CRISPR is still imperfect
While the accuracy of genetic modification through the well-known CRISPR/Cas9 system continues to improve, the technology remains imperfect. Small mistakes were initially reported [1], and later researchers found that under certain circumstances, even large swaths of DNA can be excised by mistake [2].
Obviously, this is not ready for direct use in people, and stem cells grown outside the body face a similar problem. If undesirably mutated cells are produced after CRISPR is applied, they must be removed before the modified cells could be safely used as a therapy.
Identifying which cells have the desired changes and which have undesired changes, however, is difficult, particularly when researchers are working with a mix of heterogenous cells. The solution, therefore, is to separate the individual cells and clonally grow them, creating homogenous cellular populations to analyze.
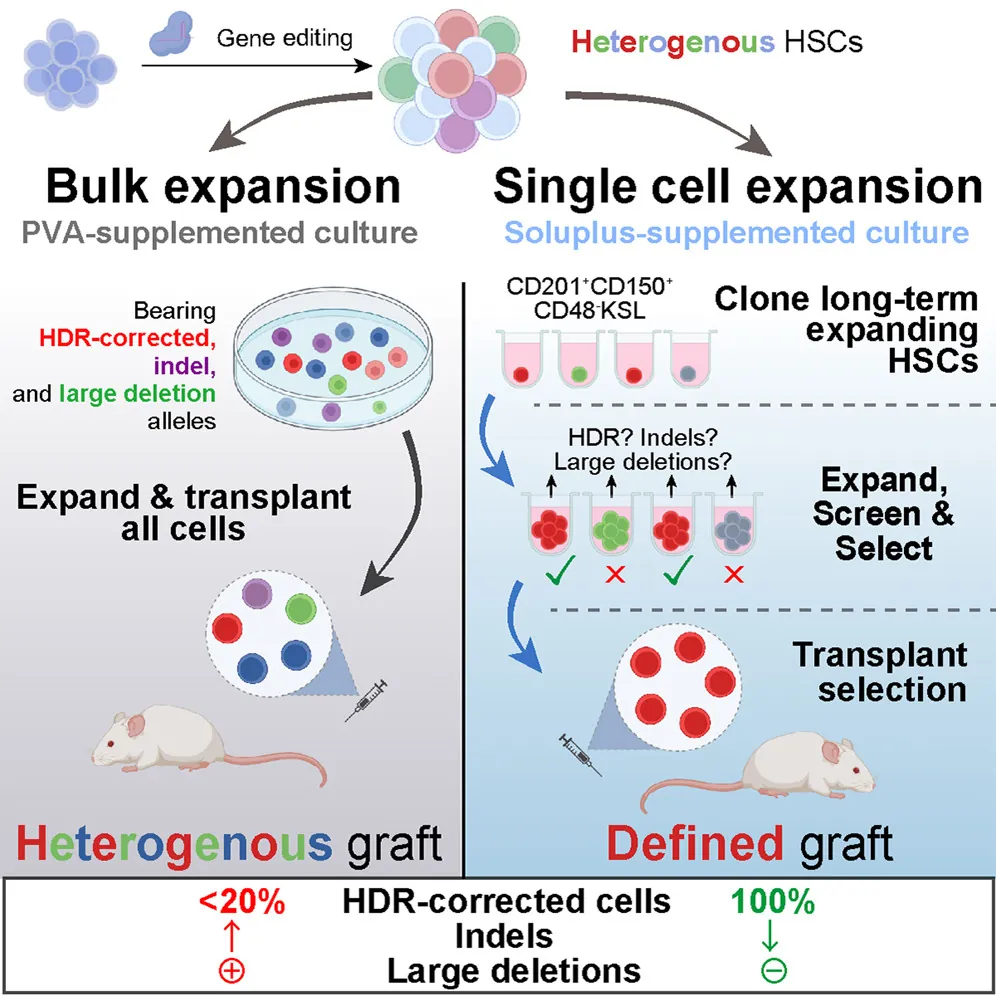
A system for expansion
Clonally expanding stem cells is not as easy as it sounds, particularly when dealing with stem cell populations that normally require signaling factors (the niche) to grow properly. These include hematopoietic stem cells (HSCs), which are the subjects of this study. These researchers have previously reported on a system that allows for the rapid expansion of HSCs in the lab [3], but only now have they taken it to the single-cell level.
The researchers attempted to grow mouse HSCs in several different media, including the polyvinyl alcohol used in their previous study [3] along with recombinant albumin and other compounds. One of these compounds was Soluplus, which is used to enhance the solubility, and hence absorption, of drugs [4]. Cells grown in 0.1% Soluplus were found to be more viable than cells grown in other compounds, and cells expressing a phenotype associated with long-term expansion were more abundant. Cytokines were also more stable in Soluplus than in other compounds.
Finally, the researchers performed the most critical test: are these cloned HSCs viable? Injecting them into mice showed that they formed largely stable grafts and that the cell populations did indeed take root without showing evidence of unwanted mutations. Interestingly, while only one of the three groups seemed to maintain these grafts, taking grafts from one mouse in which it did take root, and then injecting those cells into other mice, created entirely stable grafts in those mice.
The principle seems to work
These positive results were seen even after gene editing. Proceeding with the original plan, the researchers took a population of HSCs, subjected them to CRISPR, cloned them, and then took cells from only the clones that had successfully received modifications without off-target effects. As before, only about a third of the mice that received these stem cells had long-term stable grafts, as measured 16 weeks after injection, but taking grafts from one mouse and transplanting those into other mice produced long-term stable grafts.
The transplanted HSCs seemed to be fully functional, including in areas related to the immune system. An experimental population of mice was lacking in immune abilities, and these mice had their immune systems challenged by an antigen. The treatment group, injected with HSCs, showed evidence that these HSCs had formed T cells 19 days after injection; the control group failed to respond in the same way. Similarly, injecting human lung cancer cells into these mice formed uncontrolled tumors in the untreated mice, while the immune systems of the treated mice were significantly more effective in fighting them off.
While they did not inject any cells into human beings, the results from human cell populations were very similar. The researchers were able to winnow out undesired modifications and keep only the cell populations with the desired changes.
Hope for treating age-related diseases
If this approach can be found to work in people, it obviously has promise in treating immune disorders and other issues related to the bone marrow. It also has significant implications for aging. It may one day be feasible to take HSCs from an old person, use CRISPR/Cas9 to remove many of the genetic problems caused by genomic instability, and return that patient’s own, perfectly youthful, cells to the bone marrow.
However, even if clinical trials find this approach to be successful, it clears only one of the hurdles. For example, the stem cell niche and the overabundance of senescent cells will need to be dealt with in order to truly restore a person’s bone marrow to full, youthful functionality.
Literature
[1] Genovese, P., Schiroli, G., Escobar, G., Di Tomaso, T., Firrito, C., Calabria, A., … & Naldini, L. (2014). Targeted genome editing in human repopulating haematopoietic stem cells. Nature, 510(7504), 235-240.
[2] Boutin, J., Rosier, J., Cappellen, D., Prat, F., Toutain, J., Pennamen, P., … & Bedel, A. (2021). CRISPR-Cas9 globin editing can induce megabase-scale copy-neutral losses of heterozygosity in hematopoietic cells. Nature Communications, 12(1), 4922.
[3] Wilkinson, A. C., Ishida, R., Kikuchi, M., Sudo, K., Morita, M., Crisostomo, R. V., … & Yamazaki, S. (2019). Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature, 571(7763), 117-121.
[4] Linn, M., Collnot, E. M., Djuric, D., Hempel, K., Fabian, E., Kolter, K., & Lehr, C. M. (2012). Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. European Journal of Pharmaceutical Sciences, 45(3), 336-343.








