Metformin Reverses Myotonic Dystrophy Symptoms in Cells

A new study published in Aging shows that metformin, a drug that has been studied for its effects on longevity, restores mitochondrial function and repairs metabolic defects in cells from people with myotonic dystrophy, a condition that shares many of the same characteristics as aging.
A genetic disease that causes age-related symptoms
Myotonic dystrophy is a genetic disease that causes muscular weakness and atrophy along with multiple other disorders. As the researchers explain, it has two main types: DM1 and DM2. DM1 is both more severe and more common, and the researchers note that its effects share many similarities with the hallmarks of aging, including cellular senescence, genomic instability, telomere attrition, and deregulated nutrient sensing.
DM1 also harms the ability of mitochondria to perform their most fundamental task, which is the conversion of food into ATP; it causes one major pathway, oxidative phosphorlyzation (OXPHOS), to lose half of its ability to do this. DM1 cells also respond poorly to metabolic stresses, being largely unable to increase production when needed.
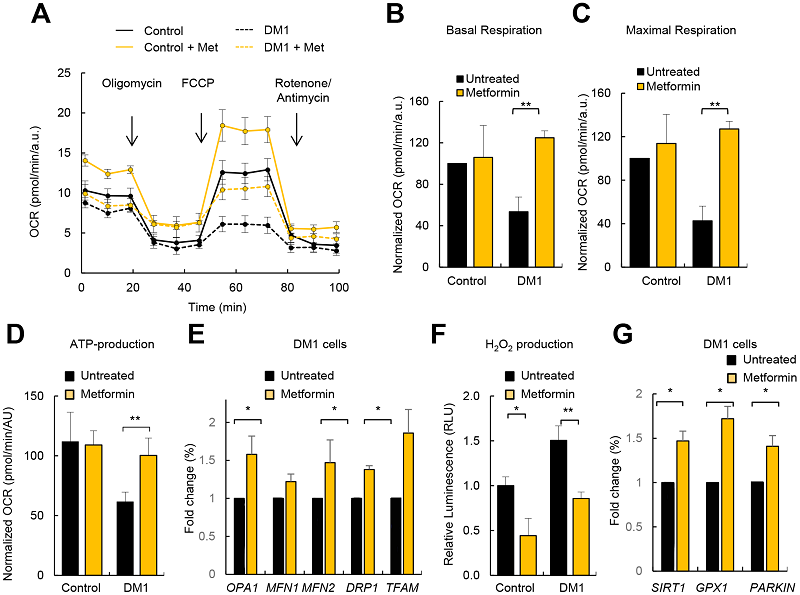
Restoring respiration
As a potential treatment for this disease, the researchers of this study turned to metformin, a drug that is known to have a beneficial influence on mitochondria [1].
The results of the study were clear. As the graphs show, the use of metformin resulted in increases of cellular respiration and ATP production close to the level of the control group, and it substantially reduced production of hydrogen peroxide, a reactive oxygen species (ROS) that leads to oxidative damage.
The study also showed that metformin increases cell division and viability in DM1 cells, decreasing markers of senescence such as p16Ink4a and p21CIP.
Abstract
Myotonic dystrophy type 1 (DM1; MIM #160900) is an autosomal dominant disorder, clinically characterized by progressive muscular weakness and multisystem degeneration. The broad phenotypes observed in patients with DM1 resemble the appearance of a multisystem accelerated aging process. However, the molecular mechanisms underlying these phenotypes remain largely unknown. In this study, we characterized the impact of metabolism and mitochondria on fibroblasts and peripheral blood mononuclear cells (PBMCs) derived from patients with DM1 and healthy individuals. Our results revealed a decrease in oxidative phosphorylation system (OXPHOS) activity, oxygen consumption rate (OCR), ATP production, energy metabolism, and mitochondrial dynamics in DM1 fibroblasts, as well as increased accumulation of reactive oxygen species (ROS). PBMCs of DM1 patients also displayed reduced mitochondrial dynamics and energy metabolism. Moreover, treatment with metformin reversed the metabolic and mitochondrial defects as well as additional accelerated aging phenotypes, such as impaired proliferation, in DM1-derived fibroblasts. Our results identify impaired cell metabolism and mitochondrial dysfunction as important drivers of DM1 pathophysiology and, therefore, reveal the efficacy of metformin treatment in a pre-clinical setting.
Conclusion
This is a cell study, not a clinical trial. While the cells used are from human beings and not mice, the results of cell studies cannot always be replicated in clinical trials. Additionally, as myotonic dystrophy is a genetic disease, the only potential true cure is a genetic one; while drugs such as metformin may be instrumental in treating the symptoms, they cannot treat the cause.
That said, however, it may be possible that metformin will offer new hope for sufferers of myotonic dystrophy or other disorders that negatively impact metabolism, and this study also helps put together disparate hallmarks of aging, such as mitochondrial dysfunction and cellular senescence.
Literature
Wang Y, An H, Liu T, Qin C, Sesaki H, Guo S, Radovick S, Hussain M, Maheshwari A, Wondisford FE, O’Rourke B, He L. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019; 29:1511–1523.e5.







