Microtubule Stabilization Ameliorates Alzheimer’s Symptoms
- The approach worked in mouse models of the disease.

A group of researchers has been able to ameliorate Alzheimer-like pathologies in mice by using a microtubule-stabilizing compound [1].
Cellular highways
Microtubules (MTs) are tiny pipe-like structures that are made mostly of the polymerized protein tubulin. They constitute an important part of the cytoskeleton, a network of filaments that permeates the cell and performs many vital functions. The cytoskeleton helps maintain the cell’s structural integrity but can also contract, changing the cell’s shape to allow for cell migration [2]. One of MTs’ most important roles is to facilitate cell division by forming spindles [3]. MTs also work as “cellular highways”; ATP-powered molecules called motor proteins, such as dynein and kinesin, literally walk along the MTs, delivering cargo (proteins and organelles) to various regions within the cell.
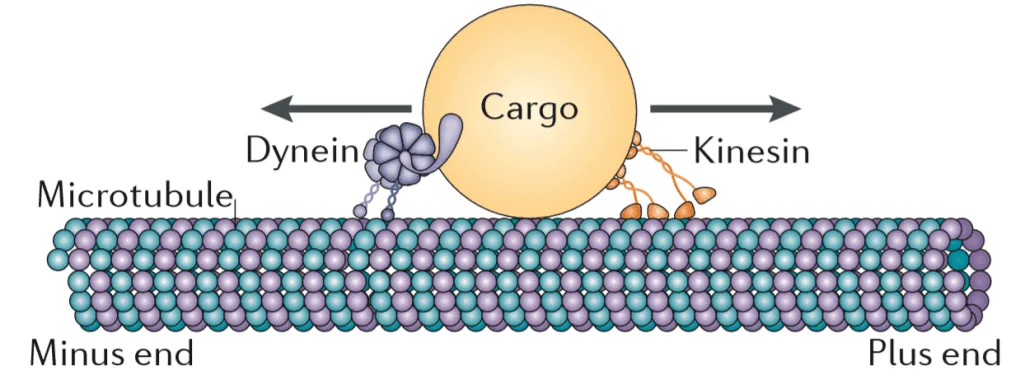
This mode of transportation is especially important for highly elongated cells such as neurons, where the body (soma) and the opposite end that contains axon terminals are connected by a long and thin axon. As a result, neurons rely on MTs for the orderly and speedy delivery of cargo to and from the soma via the axon, particularly because some axons are a full meter in length. Even the bulky energy-producing mitochondria are transported to axonal terminals via MTs.
MTs and tau proteins
Tau proteins help maintain MT stability. The problem arises when tau molecules become hyperphosphorylated, which occurs when all the locations that allow for the addition of a phosphor atom fill up. These phospho-tau molecules lose their power to hold MT components together, causing MTs to disintegrate. On top of the havoc wreaked by MT disintegration, tau molecules become clumped into insoluble aggregates called neurofibrillary tangles (NFTs). The accumulation of these tangles is a major symptom of Alzheimer’s disease (AD) and some other neurodegenerative pathologies.

Credit: Alzheimer’s Disease Education and Referral Center / National Institute on Aging
Can MT stability lead to less Aβ?
Several compounds that help maintain MT stability, such as taxanes and epothilones, are already in use in chemotherapy [4]. They have also been featured in a few recent preclinical studies of various tauopathies, including AD. What has remained unknown until now is whether they can influence the other hallmark of AD – the accumulation of amyloid-beta (Aβ) peptides.
In the current study, the researchers used transgenic mice prone to accelerated accumulation of Aβ and neuronal loss in several critically important areas of the brain, such as the hippocampus. These mice do not exhibit NFT formation but show other tau pathologies, suggesting crosstalk between tau and Aβ.
The mice that were treated with Epothilone D (EpoD), a brain-penetrating, MT-stabilizing agent, showed significant decrease in both the phospho-tau levels and in the intracellular and extracellular hippocampal Aβ accumulation. Until a few years ago, the prevalent hypothesis blamed AD development on the formation of Aβ plaques. Yet, recently, after several high-profile clinical trials based on this hypothesis ended unsuccessfully, the focus has shifted towards soluble Aβ oligomers that were found to be highly neurotoxic. Interestingly, EpoD treatment was especially effective in lowering the levels of these Aβ oligomers.
The Aβ-decreasing effect of EpoD was also confirmed in cell cultures. After ruling out other possible pathways, the researchers concluded that MT stabilization was indeed the mechanism behind Aβ reduction.
The researchers then evaluated the MT-stabilizing effect of EpoD by analyzing the expression of acetylated tubulin (AcTub). Tubulin acetylation can only happen after MTs are formed by polymerization, which makes AcTub an effective biomarker of MT stability. The untreated mice exhibited higher microtubule instability compared to the control group, but EpoD treatment brought a significant improvement on this front.
A battery of tests was performed to assess various cognitive functions in the mice, such as spatial memory and novel object recognition. EpoD-treated mice showed major improvements in most of the tests, sometimes performing similarly to the healthy control group.
MT disintegration and the consequent disruption of intracellular traffic lead to deformed and underperforming neurons. The researchers have found that EpoD helped to restore neuronal integrity and, as a result, neuronal function, which probably played a major role in the amelioration of cognitive decline.
Conclusion
This particular mechanism appears to be a positive feedback loop between Aβ accumulation and MT instability: the accelerated accumulation of Aβ in the transgenic mice apparently led to MT disintegration, which, in turn, caused even greater Aβ accumulation. The researchers were able to break this vicious circle by administering an MT-stabilizing compound that ameliorated MT disintegration and the accumulation of both Aβ and tau proteins. The treatment also resulted in healthier neurons in the vital hippocampal population and in improved cognitive function. Considering that targeting Aβ itself has been yielding mixed results, it is possible that MT stabilizers are a better way to tackle AD.
Given that AD is one of the deadliest age-related diseases, with more than 3 million new cases per year in the US alone and billions of dollars spent on AD research with few potential breakthroughs in sight, any new inroads are very welcome.
Literature
[1] Fernandez-Valenzuela, J. J., Sanchez-Varo, R., Muñoz-Castro, C., De Castro, V., Sanchez-Mejias, E., Navarro, V., … & Vizuete, M. (2020). Enhancing microtubule stabilization rescues cognitive deficits and ameliorates pathological phenotype in an amyloidogenic Alzheimer’s disease model. Scientific Reports, 10(1), 1-17.
[2] Seetharaman, S., & Etienne-Manneville, S. (2020). Cytoskeletal crosstalk in cell migration. Trends in Cell Biology.
[3] Prosser, S. L., & Pelletier, L. (2017). Mitotic spindle assembly in animal cells: a fine balancing act. Nature Reviews Molecular Cell Biology, 18(3), 187.
[4] Larkin, J. M., & Kaye, S. B. (2006). Epothilones in the treatment of cancer. Expert opinion on investigational drugs, 15(6), 691-702.







