New Peptide Puts the Brakes on Cellular Senescence
- Pep 14 reversed aspects of aging in cells and skin samples.

Researchers publishing in the Nature journal npj aging have discovered a new peptide that might prevent cells from becoming senescent and possibly youthen human skin.
Senolytics and senotherapeutics

Read More
The introduction to this paper includes a discussion of the harmful effects of senescent cells and different methods of dealing with them. Killing these cells through senolytics has become the standard method explored in research and development. However, these researchers note that as killing some senescent cell populations can lead to a loss of functions such as wound healing [1], preventing or reversing their senescence through other senotherapeutics might be a better approach [2].
Finding an effective peptide
Senotherapeutics have not been as heavily researched as senolytics, so these researchers sought to help bridge that gap by exploring a library of 164 peptides that are known not to be cytotoxic: they do not kill cells. They used fibroblast cells from a progeric human donor, more than half of which were positive for the senescence marker SA-ß-gal and had large quantities of the senescence-related enzyme ATRX [3] and senescence-related gene expression.
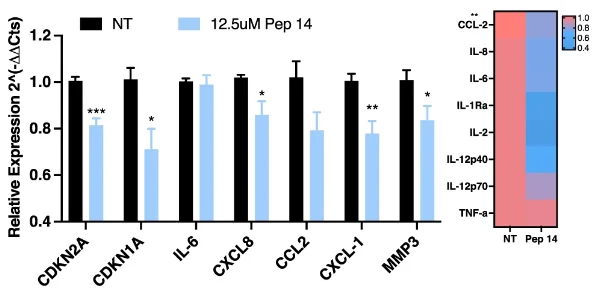
The researchers looked for the peptides that reduced the percentage of senescent cells, then re-analyzed to create a different database of 764 potential candidates. Of this large group, the researchers selected five with the least toxicity and greatest senotherapeutic potential. The best-performing one was Pep 14, a compound that does not closely resemble any other known protein and that OneSkin claims to use in its OS-01 line of products. It was found to reduce multiple senescent markers, although gene expression (left) and an ELISA protein assay (right) did not fully agree with each other.
Pathways and tissue samples
A closer look at RNA pathways found that Pep 14 reversed many of the epigenetic alterations associated with aging. Signaling, longevity, and cellular senescence pathways were all affected in positive ways, which, interestingly, were largely different from rapamycin’s effects. Pep 14 was found to modulate part of the PP2A pathway, which these researchers discovered to be part of cellular senescence.
A close examination of cellular populations found that Pep 14 delayed and discouraged the transition into late senescence, decreasing their proportions in samples. However, it was not found to have any affect on autophagy nor any senolytic properties: it does not kill cells.
Samples of donor skin and 3D-grown skin tissues showed encouraging results even compared to rapamycin. Samples treated with Pep 14 had more organized layers and better skin thickness than rapamycin-treated or untreated samples, and aging biomarkers were also improved. A formula designed to sink into the skin was developed, and it was found to perform better than retinol, which is often used to treat skin aging.
Promising, but early stages
These experiments were performed in skin cells and tissue samples, not mice and certainly not people. It remains to be seen whether Pep 14 can be safely and effectively administered to living organisms at all and if it has effects on other parts of the body. However, these results show significant potential, and we look forward to preclinical experiments and possibly clinical trials of this promising compound.
OneSkin, which funded this study and whose founders led it, is a corporate sponsor of Lifespan.io.Literature
[1] Demaria, M., Ohtani, N., Youssef, S. A., Rodier, F., Toussaint, W., Mitchell, J. R., … & Campisi, J. (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell, 31(6), 722-733.
[2] Kim, E. C., & Kim, J. R. (2019). Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB reports, 52(1), 47.
[3] Kovatcheva, M., Liao, W., Klein, M. E., Robine, N., Geiger, H., Crago, A. M., … & Koff, A. (2017). ATRX is a regulator of therapy induced senescence in human cells. Nature communications, 8(1), 386.








