Results of a Crowdfunded One-Year Human Rapamycin Trial
- The benefits were much stronger for women than men.

In Aging, Dr. Sajid Zalzala and his team have published the results of Participatory Evaluation of Aging with Rapamycin for Longevity (PEARL), a randomized, controlled human clinical trial that was crowdfunded by Lifespan.io.
Crowdfunded research bears fruit
Four years ago, in collaboration with AgelessRx, Lifespan.io crowdfunded the PEARL trial, raising $182,838 and significantly overshooting its donation goal of $75,000. This support was greatly appreciated, but in a way, it was unsurprising: rapamycin is a very popular subject in longevity, being one of the most well-known and most thoroughly researched compounds in this space. In fact, this compound’s principal target is known as the mammalian target of rapamycin (mTOR) [1]. Its life-extending benefits are related to Complex 1 of this target (mTORC1) [2]; mTORC2, which is a useful target for cancer but is not a desirable target in aging, is less sensitive to rapamycin.
Most of the previous work on rapamycin’s potential for life extension was conducted on animals; mTOR inhibition has been found to extend lifespan in mice [3] and monkeys [4]. However, despite that research, and the fact that rapamycin is already an FDA-approved drug for immunosuppression, there has been relatively little research into whether or not it can truly extend human lifespan or healthspan. Most previous work in this area was done in short durations and focused on specific aspects of aging, such as the immune system [5].
These researchers, therefore, sought to fill this gap by devising PEARL, which they described as the first long-running randomized controlled trial of rapamycin in humans. Over a period of 48 weeks, they administered rapamycin at 5 and 10 milligrams to two different groups along with a placebo control; these are the doses that are normally taken off-label by longevity enthusiasts and are much lower than the immunosuppressive dose. Importantly, in order to better facilitate the existence of a control group, they used compounded rapamycin, but they paused the trial and found it to be only a third as effective as introducing rapamycin into the blood than the commercial variety, sirolimus [6].
Generally safe with mixed benefits
In total, 114 people with an average age of approximately 60 years completed this study: 35 in the 10 mg group, 40 in the 5 mg group, and 39 in the control group. Only 40 of the participants were women. Adverse events, including significant adverse events, were not different between the groups, suggesting that these doses of rapamycin are generally safe. Most metrics of body composition were unaffected over the 48 weeks, including visceral fat mass, which was the researchers’ primary endpoint.
Red blood cell counts did increase in the 5 mg group, and blood carbon dioxide decreased in the 10 mg group. Men in the 10 mg group had decreased blood calcium and an increase in blood urea nitrogen (BUN), potentially suggesting a kidney-related concern, and men in the 5 mg group had slightly increased A1C, a potential marker of diabetes.
Only women experienced most of the benefits, despite there being fewer women in this study than men. Women in the 10 mg group who took rapamycin had significantly increased lean tissue mass and reported reduced amounts of pain. Interestingly, only the 5 mg group saw benefits for general health, but this included both women and men. Emotional well-being was improved in the 5 mg group after 48 weeks, but it was improved in the placebo group as well.
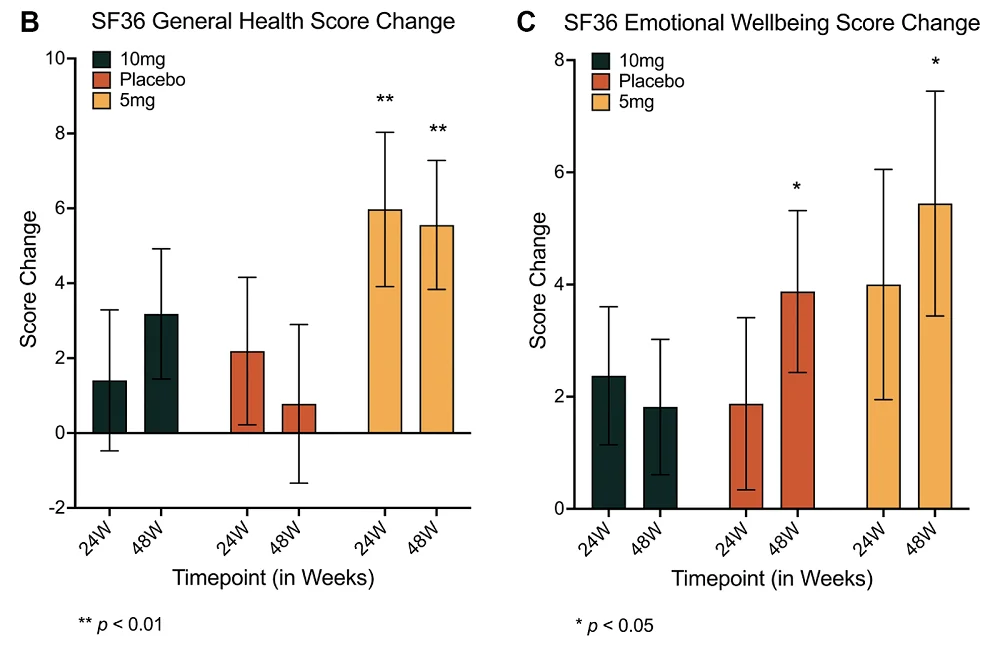
“In general, the PEARL trial showed that female participants had the greatest benefits overall, with women in the higher-dose group seeing significant improvements in lean muscle mass and improvements in self-reported pain,” said Dr. Sajad Zalzala, CEO of AgelessRX and lead author of this study. “This outcome was particularly meaningful, as the study was powered primarily to assess safety and tolerability in a small pilot cohort rather than efficacy.”
Rapamycin showed no benefits over placebo in other self-reported metrics, such as physical function and social interactions. Osteoarthritis, as measured by WOMAC, was also unaffected.
This study showed a potential side effect of gut problems. A subgroup of participants (50 females, 31 males) had their gut microbiomes tested: in males, markers of gut dysbiosis increased in the 10 mg group, and in females, there was a trend towards increased permeability of the intestines.
A subgroup of 15 male and 9 female participants also underwent epigenetic aging analysis, performed using the commercially available test TruAge from the company TruDiagnostics. Unfortunately, there were no statistically significant effects in this area.
“This study adds to a growing body of evidence indicating that rapamycin can be used safely in relatively healthy individuals,” said Matt Kaeberlein, former professor at the University of Washington, CEO of Optispan, and co-founder of Ora Biomedical. “That alone is an important step forward. I’d say we’re seeing consistent anecdotal signals – supported now by the PEARL data – that rapamycin may help preserve lean mass and potentially improve quality of life, particularly in women. That’s promising, but due to low dosing in this study and limited sample size, not yet definitive.”
Future studies
These researchers note that this study was conducted on a mixed group of participants who are largely longevity-conscious, and they suggest that rapamycin may have stronger effects on less healthy people. Despite the low efficacy of compounded rapamycin compared to sirolimus, there were still significant effects on lean tissue mass and pain in women. The researchers hold that these statistically significant benefits, despite the low number of people involved, suggest that rapamycin does indeed have significant longevity benefits.
This trial relied largely on self-reporting and did not provide direct evidence that rapamycin use extends the lives of humans, and most of its effects on long-term health were limited. To directly make the claim that rapamycin improves lifespan or healthspan, a much larger and much longer trial would have to be conducted. Such a trial may use a compound that only affects mTORC1 (a rapalog) or different doses of rapamycin.
Still, PEARL was a significant step forward in studying the use of rapamycin for staving off aging in humans. “We are delighted to have supported the PEARL clinical trial back in 2021 and are excited to see the data from it appearing in academic publications,” said Keith Comito, founder of Lifespan.io, which has since merged with SENS Research Foundation to become the Lifespan Research Institute. “Trials like PEARL are an important step towards a deeper understanding of what aging is and what drives it. Ultimately, through crowdfunding and conducting research directly, we can all do our part in overcoming age-related diseases, together.”
Literature
[1] Mannick, J. B., & Lamming, D. W. (2023). Targeting the biology of aging with mTOR inhibitors. Nature Aging, 3(6), 642-660.
[2] Castilho, R. M., Squarize, C. H., Chodosh, L. A., Williams, B. O., & Gutkind, J. S. (2009). mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell stem cell, 5(3), 279-289.
[3] Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., … & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. nature, 460(7253), 392-395.
[4] Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., Beasley, T. M., … & Weindruch, R. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science, 325(5937), 201-204.
[5] Mannick, J. B., Morris, M., Hockey, H. U. P., Roma, G., Beibel, M., Kulmatycki, K., … & Klickstein, L. B. (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Science translational medicine, 10(449), eaaq1564.
[6] Harinath, G., Lee, V., Nyquist, A., Moel, M., Wouters, M., Hagemeier, J., … & Zalzala, S. (2025). The bioavailability and blood levels of low-dose rapamycin for longevity in real-world cohorts of normative aging individuals. GeroScience, 1-14.








