Senescence May Play a Significant Role in Parkinson’s
- Lipid accumulation appears to be the driving factor.

In Aging, a pair of researchers has published a perspective connecting fat (lipid) accumulation and cellular senescence in neurons to Parkinson’s disease.
α-syn, but not just α-syn
Parkinson’s disease is characterized by the loss of a specific population of neurons: the dopaminergic neurons in the substantia nigra, a part of the brain that governs movement [1]. The ensuing problems with basic movement are accompanied by cognitive decline and depression.
At the cellular level, a key hallmark is the accumulation of alpha-synuclein (α-syn) aggregates that lead to Lewy bodies. This is connected to lipid metabolism, and research has found that stressors that encourage Parkinson’s disease also encourage lipid accumulation [2]. Aging is, of course, the strongest risk factor for Parkinson’s, but genetic factors also play a role: the most well-documented of these is a mutation of GBA, a gene that encodes for β-glucocerebrosidase (GCase), an enzyme that breaks down glucosylceramides (GluCer), a class of lipids.
This relationship appears to be a strong one: increased amounts of GluCer are associated with sharp cognitive decline in Parkinson’s patients, and a lack of GCase appears to be the cause [3]. Dysfunction of the lysosomes [4], which break down protein, and reactions with dopamine itself [5] may also contribute to this increase in GluCer.
The genetic link
These researchers had found that this is also connected to cellular senescence [6]. SATB1, a gene that has also been found to be a risk factor for Parkinson’s, downregulates the microRNA miR-22-3p, which downregulates GBA. Therefore, a reduction in SATB1 leads to more GluCer and senescence in the neurons. In that paper, these researchers had treated human neurons with GluCer and found that it drove them senescent and encouraged α-syn aggregation. A previous paper had also found that lipid droplet accumulation and cellular senescence are connected [7].
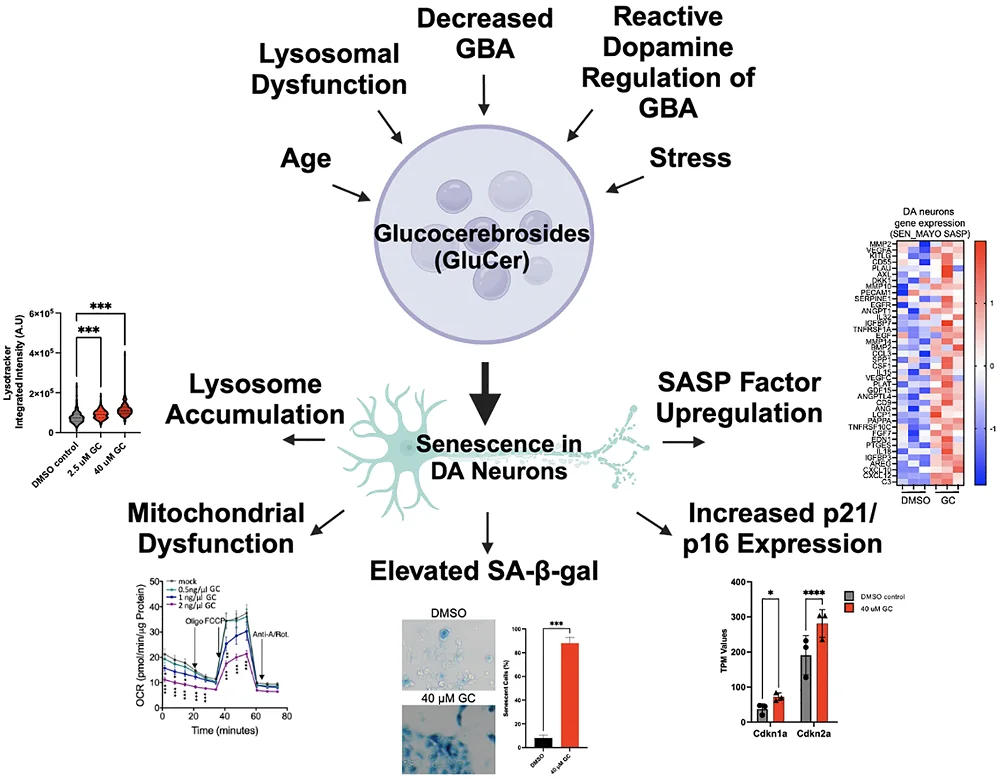
This connection between lipid droplets and senescence also appears to involve α-syn. Lipid droplets themselves encourage its production, and α-syn by itself has been found to drive the relevant cells senescent [8]. The researchers note that this may be dependent on cell type: a reduction in SATB1 drives dopaminergic neurons, which are specifically harmed by Parkinson’s disease, senescent [9], while it does not drive other types of neurons senescent. These neurons, in particular, are dependent on the function of lysosomes.
This difference in specific cell type appears to be why Parkinson’s is limited to such a small and critical population of neurons. Most critically, it gives researchers a potential new target for Parkinson’s therapies. If GBA or lipid accumulation can be directly affected in addition to α-syn, it might be possible for Parkinson’s disease to be treated much more effectively.
Literature
[1] Dauer, W., & Przedborski, S. (2003). Parkinson’s disease: mechanisms and models. Neuron, 39(6), 889-909.
[2] Alecu, I., & Bennett, S. A. (2019). Dysregulated lipid metabolism and its role in α-synucleinopathy in Parkinson’s disease. Frontiers in neuroscience, 13, 328.
[3] Huh, Y. E., Park, H., Chiang, M. S. R., Tuncali, I., Liu, G., Locascio, J. J., … & Scherzer, C. R. (2021). Glucosylceramide in cerebrospinal fluid of patients with GBA-associated and idiopathic Parkinson’s disease enrolled in PPMI. npj Parkinson’s Disease, 7(1), 102.
[4] Arévalo, N. B., Lamaizon, C. M., Cavieres, V. A., Burgos, P. V., Álvarez, A. R., Yañez, M. J., & Zanlungo, S. (2022). Neuronopathic Gaucher disease: Beyond lysosomal dysfunction. Frontiers in Molecular Neuroscience, 15, 934820.
[5] Riessland, M., Kolisnyk, B., & Greengard, P. (2017). Reactive dopamine leads to triple trouble in nigral neurons. Biochemistry, 56(49), 6409-6410.
[6] Russo, T., Kolisnyk, B., BS, A., Plessis‐Belair, J., Kim, T. W., Martin, J., … & Riessland, M. (2024). The SATB1‐MIR22‐GBA axis mediates glucocerebroside accumulation inducing a cellular senescence‐like phenotype in dopaminergic neurons. Aging cell, 23(4), e14077.
[7] Millner, A., & Atilla-Gokcumen, G. E. Lipid Players of Cellular Senescence. Metabolites 2020, 10, 339.
[8] Verma, D. K., Seo, B. A., Ghosh, A., Ma, S. X., Hernandez-Quijada, K., Andersen, J. K., … & Kim, Y. H. (2021). Alpha-synuclein preformed fibrils induce cellular senescence in Parkinson’s disease models. Cells, 10(7), 1694.
[9] Riessland, M., Kolisnyk, B., Kim, T. W., Cheng, J., Ni, J., Pearson, J. A., … & Greengard, P. (2019). Loss of SATB1 induces p21-dependent cellular senescence in post-mitotic dopaminergic neurons. Cell stem cell, 25(4), 514-530.







