Senescent Cells Promote Cartilage Regeneration in Rats
- Here, the SASP encourages cells to divide.
In a rat experiment, researchers publishing in Aging Cell have found that senescent cells and SASP factors are key in regenerating knee cartilage.
Not always negative
Cellular senescence is widely known to have negative effects, to the point that it is one of the hallmarks of aging. In fact, rather than protecting cartilage, cellular senescence has been reported to damage it in the progression of osteoarthritis [1]. However, the idea that senescence is beneficial for regeneration is not a new concept [2], and it has been found to assist wound healing in mice [3]. Understanding everything involved in this complex relationship is not easy, and one of the factors appears to be windows of time [4].
The authors refer to the beneficial effects as originating from a transient accumulation and a “SASP burst”, citing studies that link it to the exceptional regeneration of the zebrafish [5] and the axolotl [6].
The problem with healing the meniscus and other cartilaginous tissues is that they are avascular: they do not have blood vessels, so functional cells that can repair the area are not normally present. This makes it easier for mechanical damage to wear them down over time [7]. However, the surrounding synovium has been found to regenerate it in mice [8].
When senescence and regeneration coincide
With this prior work in mind, the researchers decided to take a closer look at the meniscus by using 53 male Lewis rats. Some of these rats received a sham surgery that only opened and closed the joint, another group had half of the meniscus of each rear leg removed, and another group received no surgery at all as controls.
One week after the true surgery, the animals were well into regenerating the damaged area, with new, irregular tissue appearing from the synovium. The macrophage factor CD68 and the well-known senescence marker p16 were strongly expressed in this regenerative tissue compared to the rest of the meniscus and the synovium.
However, their presence dropped dramatically a week after that, and by three months after the surgery, there was no discernible difference: the new tissue was expressing the same factors as the rest of the meniscus. Even the sham surgery was found to slightly increase these factors in the synovium. The senescence indicator SA-β-gal was similar, with staining being clearly visible in the sham group and even more visible in the surgery group.
Gene expression analysis found that the pro-regeneration factor SOX9 (not to be confused with the OSKM factor SOX2) was being upregulated alongside Cdkn2a, the gene that codes for p16. The cells were not the same ones: instead, p16-expressing cells appeared to be encouraging nearby cells to express SOX9, suggesting that something that they were emitting was encouraging regeneration.
The benefits of the SASP
The researchers then confirmed that the SASP was indeed beneficial for these fibroblasts. Administering senescence-inducing factors to fibroblasts in culture caused them to send out their own signals. Exposing other fibroblasts to those signals in a cultured medium did not drive them senescent, as the SASP is often reported to do: instead, it caused them to rapidly proliferate, with numbers being much higher than a control group.
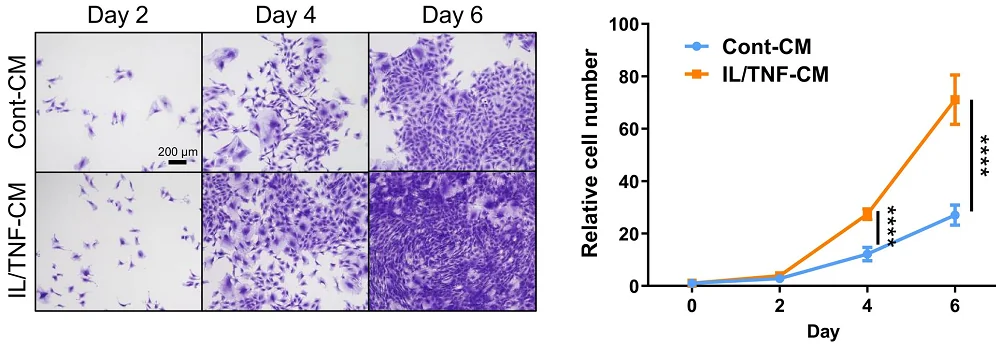
The necessity of senescent cells was confirmed in another rat experiment. The senolytic compound ABT-263 was given to a population of rats 1 to 4 weeks after the meniscus surgery. The senolytic was effective, with multiple senescence biomarkers reduced by roughly half. There was less healthy cartilage regenerated in this group compared to the surgery-only group, and SOX9 was similarly decreased.
While the SASP is often condemned for being a driver of age-related diseases, this experiment makes it clear that it has beneficial effects as well. Future experiments to confirm that senescent cells are just as necessary for regeneration in both sexes and in other animals may be necessary. Clearly, a more thorough understanding of senescent cells’ effects on multiple tissues is required before senolytic treatments become widespread in the clinic, lest such treatments do more harm than good.
Literature
[1] Coryell, P. R., Diekman, B. O., & Loeser, R. F. (2021). Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nature Reviews Rheumatology, 17(1), 47-57.
[2] Serrano, M. (2014). Senescence helps regeneration. Developmental cell, 31(6), 671-672.
[3] Demaria, M., Ohtani, N., Youssef, S. A., Rodier, F., Toussaint, W., Mitchell, J. R., … & Campisi, J. (2014). An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell, 31(6), 722-733.
[4] de Carvalho, D. P., & Jacinto, A. (2021). The right time for senescence. Elife.
[5] Da Silva‐Álvarez, S., Guerra‐Varela, J., Sobrido‐Cameán, D., Quelle, A., Barreiro‐Iglesias, A., Sánchez, L., & Collado, M. (2020). Cell senescence contributes to tissue regeneration in zebrafish. Aging cell, 19(1), e13052.
[6] Yu, Q., Walters, H. E., Pasquini, G., Singh, S. P., Lachnit, M., Oliveira, C. R., … & Yun, M. H. (2023). Cellular senescence promotes progenitor cell expansion during axolotl limb regeneration. Developmental cell, 58(22), 2416-2427.
[7] Bansal, S., Floyd, E. R., A Kowalski, M., Aikman, E., Elrod, P., Burkey, K., … & Patel, J. M. (2021). Meniscal repair: the current state and recent advances in augmentation. Journal of Orthopaedic Research®, 39(7), 1368-1382.
[8] Hiyama, K., Muneta, T., Koga, H., Sekiya, I., & Tsuji, K. (2017). Meniscal regeneration after resection of the anterior half of the medial meniscus in mice. Journal of Orthopaedic Research, 35(9), 1958-1965.








