Senolytic Helps Anti-Osteoarthritis Potential in Stem Cells
- Reducing the SASP allows stem cells to thrive.

Scientists have improved the potential effectiveness of a stem cell therapy for osteoarthritis by targeting senescent cells [1].
Stem cell exhaustion and osteoarthritis
Osteoarthritis is an age-related disease that affects joints by degrading cartilage. While not fatal in itself, osteoarthritis negatively affects mobility and quality of life, and it can decrease lifespan and healthspan. Several studies link osteoarthritis to higher mortality, especially from cardiovascular diseases [2]. Osteoarthritis patients may require drastic measures such as knee replacement, while less invasive treatments have limited effectiveness.
Osteoarthritis can be viewed as a loss of balance between the degradation and regeneration of joint cartilage. This is partially caused by the age-related exhaustion of mesenchymal stem cells (MSCs) that are supposed to differentiate into healthy cartilage-producing cells (chondrocytes). Emerging stem cell therapies for osteoarthritis work by harvesting MSCs from the patient, culturing them in vitro to increase their number and injecting them directly into the affected joint [3].
These therapies, already available in several countries, though not approved in the US, are still limited by the age-related decline in MSC function. Producing brand-new MSCs from induced pluripotent stem cells (iPSCs), which undergo rejuvenation during reprogramming, is a promising direction [3] but is still years away from clinical use.
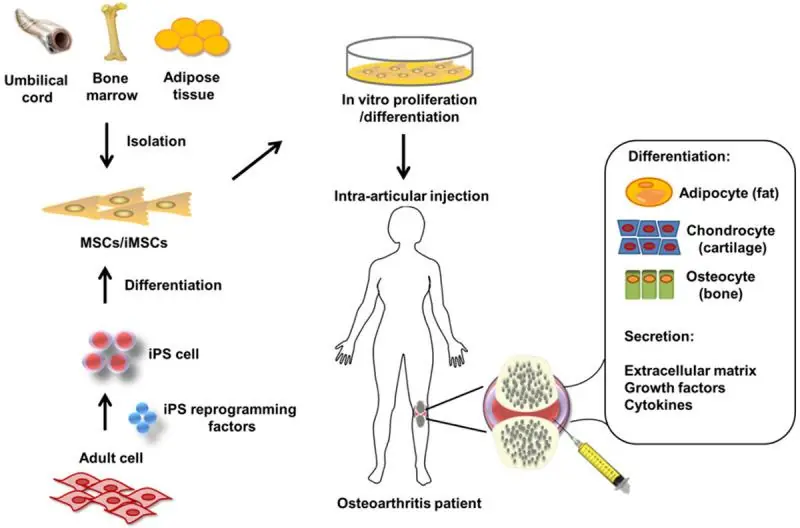
High levels of senescence
In this new study, Japanese scientists discovered that MSCs derived from osteoarthritis patients contain a large fraction of senescent cells, which might be an important cause of the decline in their regenerative potential. Not only are senescent stem cells unable to differentiate into chondrocytes, but they also damage neighboring stem cells by secreting the senescensce-associated secretory phenotype (SASP), a cocktail of mostly pro-inflammatory molecules.

Read More
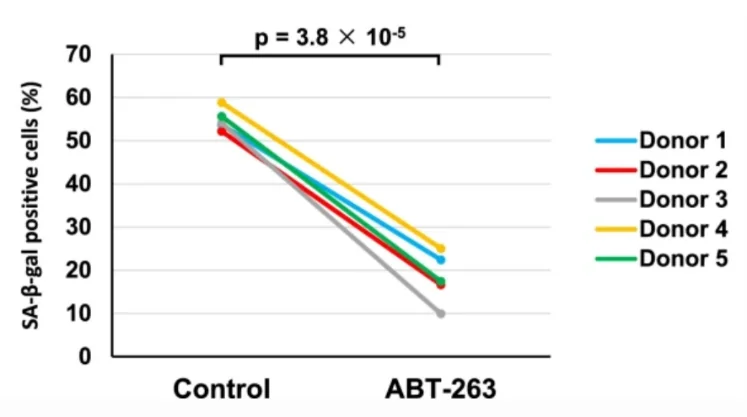
In search of a remedy, the researchers derived synovial MSCs from five donors and treated them with the senolytic drug ABT-263 for one day. Seven days later, senescence levels, colony-forming potential, and multipotency were assessed. The average percentage of senescent (β-galactosidase-positive) cells at baseline was a whopping 55%. Treatment with ABT-263 lowered this percentage to 18% in a uniform manner across all samples:
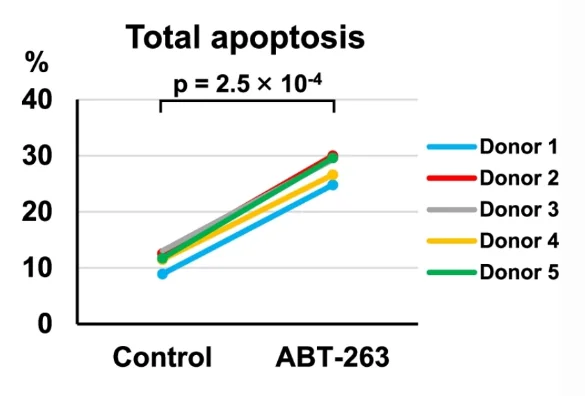
The researchers then confirmed that ABT-263 cleared senescent cells by inducing apoptosis (cellular death). This was done by assessing the levels of caspase-3, a central element of the apoptotic pathway:
MSC therapy works by harvesting MSCs and making them grow into colonies. In the study, the researchers assessed colony-forming potential by culturing the cells for 14 days. Cells treated with ABT-263 produced many more colonies, and the colonies were significantly larger than those produced by controls. In the culture, the researchers found enlarged and flattened cells that did not produce colonies. This morphology is characteristic of senescent cells. The percentage of those dysfunctional cells was much lower in the treated group, in line with the hypothesis that ABT-263 eliminates senescent cells.
Improved regenerative potential
Treated cells were also much better than controls in forming cartilaginous pellets, which is a measure of MSCs’ ability to differentiate into chondrocytes and regenerate cartilage. The mean pellet weight was more than twice as large in the treatment group, and the pellets were richer in essential ECM elements such as glycosaminoglycan. The treated cells also produced the “right” collagen: there was less collagen type I, which is mostly present in the skin, tendons, ligaments, and bones, and more collagen type II, the staple protein in cartilage.
As expected, the treatment also decreased the production of SASP, including the collagen-degrading enzyme MMP-13 and the pro-inflammatory cytokine IL-6.
Conclusion
This proof-of-concept study shows a way to increase the regenerative potential of stem cells, which might not be limited to treating osteoarthritis. It also highlights the prevalence of cellular senescence in stem cells from aging donors as one of the problems that must be solved on the way to developing effective stem cell therapies for age-related diseases. As the researchers note, one of the limitations of their study was that the therapeutic effect of the senolytic treatment was not investigated in vivo. Hopefully, follow-up studies will be able to address this question.
Literature
[1] Miura, Y., Endo, K., Komori, K., & Sekiya, I. (2022). Clearance of senescent cells with ABT-263 improves biological functions of synovial mesenchymal stem cells from osteoarthritis patients. Stem Cell Research & Therapy, 13(1), 1-15.
[2] Veronese, N., Cereda, E., Maggi, S., Luchini, C., Solmi, M., Smith, T., … & Stubbs, B. (2016, October). Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. In Seminars in arthritis and rheumatism (Vol. 46, No. 2, pp. 160-167). WB Saunders.
[3] Zhu, C., Wu, W., & Qu, X. (2021). Mesenchymal stem cells in osteoarthritis therapy: A review. American journal of translational research, 13(2), 448.








