A New Anti-Inflammatory Approach for Arthritis
- This compound directly counteracts an inflammatory cytokine.
In Aging, researchers have published their tests of a compound that counteracts the effects of tumor necrosis factor alpha (TNF-α), an inflammatory factor that promotes arthritis.
Current anti-inflammatories aren’t enough
Rheumatoid arthritis, an autoimmune disorder that causes joint swelling, pain, and disability, is most often treated with anti-inflammatory compounds, such as non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and more modern biological treatments [1]. Many of these approaches have serious side effects: NSAIDs can cause gastrointestinal damage [2], and some treatments can cause immunodeficiencies and liver damage [3]. Overall, current treatments for arthritis are of only limited effectiveness [4].
This team sought a better approach by focusing on fibroblast-like synoviocytes (FLS), which are found in the lining of joints. These cells can behave like cancerous tumor cells, migrating into and invading other tissues in ways that ordinary fibroblasts do not [5]. The excessive growth of individual FLS cells is a significant part of rheumatoid arthritis [6], as is their senescence [7].
To counteract this, these researchers examined phoenixin, a natural compound that has been found in multiple mammalian tissues and found to exhibit anti-inflammatory properties [8].
Restoring a vital protein to reduce senescence
Phoenixin directly targets GPR173, a protein that is downregulated by TNF-α, as confirmed by these researchers’ first experiment. The researchers then tested the cellular safety of phoenixin, culturing cells in various concentrations. It was found not to affect FLS viability after 20 nanomoles (nM).
They then administered phoenixin to cells that had been also been cultured with high levels of TNF-α. After one day, cells not given phoenixin had other inflammatory factors, such as IL-6, IL-8, and IL-1ß, dramatically upregulated. Increasing concentrations of phoenixin reduced these factors in a dose-dependent manner.
Phoenixin was also found to reduce cellular senescence. FLS given phoenixin had significantly reduced levels of the senescence biomarkers SA-ß-gal, STAT6, p53, and p21. To confirm the molecular mechanism, the researchers also increased STAT6 through a lentivirus, which neutralized the effects of phoenixin. Telomerase, which is crucial for cellular division and is negatively associated with senescence, was restored by phoenixin.
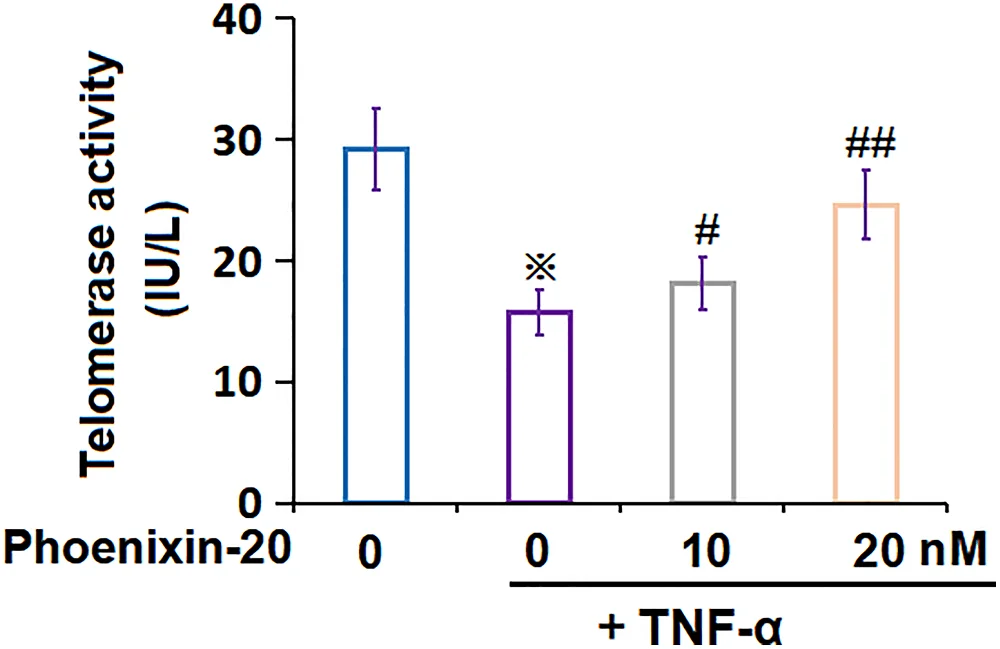
The researchers describe phoenixitin as a compound that protects FLS against senescence and reduces the production of inflammatory cytokines, which are known to be excreted by senescent cells and induce further senescence. This makes it a potential treatment for rheumatoid arthritis.
However, these results were in specific cells, not living organisms. While it does exist in certain tissues, phoenixin may be toxic or ineffective when administered to animals. Further preclinical experiments will have to be done before this compound can be tested in human beings.
Literature
[1] Adami, G., Orsolini, G., Adami, S., Viapiana, O., Idolazzi, L., Gatti, D., & Rossini, M. (2016). Effects of TNF inhibitors on parathyroid hormone and Wnt signaling antagonists in rheumatoid arthritis. Calcified tissue international, 99, 360-364.
[2] Roth, S. H. (2012). Coming to terms with nonsteroidal anti-inflammatory drug gastropathy. Drugs, 72, 873-879.
[3] Chatzidionysiou, K., Emamikia, S., Nam, J., Ramiro, S., Smolen, J., van der Heijde, D., … & Landewé, R. (2017). Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Annals of the rheumatic diseases, 76(6), 1102-1107.
[4] Schultz, M., Keeling, S. O., Katz, S. J., Maksymowych, W. P., Eurich, D. T., & Hall, J. J. (2017). Clinical effectiveness and safety of leflunomide in inflammatory arthritis: a report from the RAPPORT database with supporting patient survey. Clinical Rheumatology, 36, 1471-1478.
[5] Bottini, N., & Firestein, G. S. (2013). Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nature Reviews Rheumatology, 9(1), 24-33.
[6] Huber, L. C., Distler, O., Tarner, I., Gay, R. E., Gay, S., & Pap, T. (2006). Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology, 45(6), 669-675.
[7] Chen, X., Gong, W., Shao, X., Shi, T., Zhang, L., Dong, J., … & Guo, B. (2022). METTL3-mediated m6A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Annals of the rheumatic diseases, 81(1), 85-97.
[8] Sun, G., Ren, Q., Bai, L., & Zhang, L. (2020). Phoenixin-20 suppresses lipopolysaccharide-induced inflammation in dental pulp cells. Chemico-Biological Interactions, 318, 108971.







