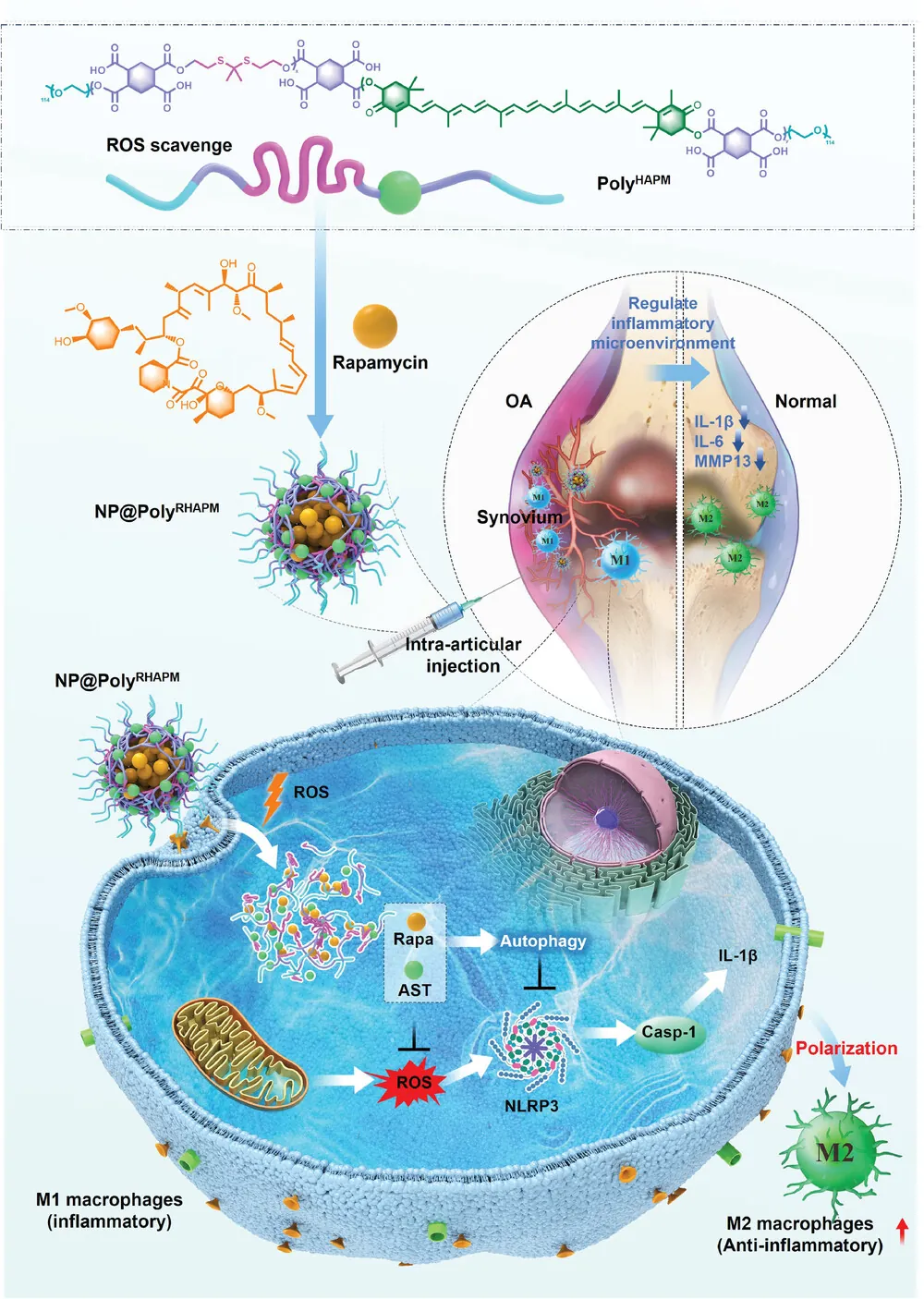
Researchers have described how specialized nanoparticles, which deliver rapamycin and the antioxidant astaxanthin, restore macrophage balance and reduce inflammation in a mouse model of osteoarthritis.
A matter of polarization
Inflammation is a critical part of arthritis. Previous studies have found that inflammation of the membranes in joints (synovitis) strongly contributes to osteoarthritis [1]; in fact, even without mechanical joint damage, synovitis can lead to this crippling disease [2].
Macrophage polarization has a profound effect on inflammation. M1-polarized macrophages are responsible for generating inflammation, such as interleukins and TNF-α, in response to threats [3]; the M2 polarization is responsible for the healing and repair afterwards, and it can do so in joints [4]. However, in arthritis and many other age-related diseases, the inflammatory state sticks around. This is known as inflammaging.
Delivering the anti-inflammatories
Astaxanthin is a naturally occurring antioxidant and anti-inflammatory compound that is commonly used for treating inflammatory diseases [5]. The Interventions Testing Program (ITP) has found that astaxanthin extends the lives of healthy mice.
Rapamycin needs little introduction, as it is one of the most well-known longevity-related compounds and has also been found to extend the lives of mice in the ITP. Among its many metabolic effects, rapamycin has been reported to spur the cellular maintenance process of autophagy and subsequently reduce inflammation [6].
We have previously reported on a study in which extracellular vesicles are only released from a hydrogel in the presence of reactive oxygen species (ROS). This researchers have followed a somewhat similar approach, creating nanoparticles that enter cells and burst open only in the presence of ROS to deliver a payload containing astaxathin and rapamycin. The exterior filaments of the nanoparticle themselves neutralize ROS, the astaxathin is attached to those filaments, and the rapamycin is clustered inside.
Effects in macrophages
Further testing revealed that this nanoparticle is taken up by macrophages and is effective inside them. The researchers used lipopolysaccharide to irritate macrophages into the M1 state, then applied their new compound and its payload. Althouth this nanoparticle is toxic to macrophages in higher doses, the combined payload of nanoparticle and rapamycin was found to reduce ROS to a small fraction of that of an M1 control group.
Similarly, inflammatory interleukins were reduced by this novel nanoparticle. The inflammation in macrophages also affects their own function. Levels of naturally occurring antioxidants were increased by the treatment, markers of autophagy were improved, and the membrane potential of mitochondria was restored. Overall, biomarkers of inflammation were significantly ameliorated in the targeted macrophages.
Most critically, delivering the full payload to M1 macrophages decreased biomarkers of M1 by nearly four times and increased biomarkers of M2 by approximately the same amount. Therefore, according to the researchers, this nanoparticle successfully reprogrammed macrophages from the M1 to the M2 type.
While conditioned media taken from M1 macrophages can be deadly to cartilage cells (chondrocytes) when exposed over time, decreasing their viability and slowing their growth, conditioned media taken from the treated macrophages was substantially less dangerous, halving the rate of cellular death by apoptosis.
Reduces arthritis in mice
The researchers then turned to a model of osteoarthritis in mice. Applying the rapamycin-containing nanoparticle to these animals significantly decreased the swelling in their joints. The treatment was found to substantially protect their cartilage, preventing joint deterioration and putting a lid on inflammation.
Mice given this nanoparticle had substantially downregulated M1 biomarkers and correspondingly upregulated M2 biomarkers, just like in the cellular study. While the nanoparticle is processed by the liver and needed to be readministered every three days to retain its presence, no liver toxicity was found.
Being able to reprogram macrophages away from M1 and into M2 represents a significant milestone for anti-inflammatory therapies and the treatment of age-related diseases as a whole, with implications that go beyond arthritis. However, this is a cellular and mouse study, and the effectiveness and potential side effects haven’t been tested in human beings. A clinical trial would need to be done to determine if this is truly a revolutionary approach.
Literature
[1] Li, M., Li, H., Ran, X., Yin, H., Luo, X., & Chen, Z. (2021). Effects of adenovirus-mediated knockdown of IRAK4 on synovitis in the osteoarthritis rabbit model. Arthritis Research & Therapy, 23, 1-12.
[2] Goldring, M. B., & Otero, M. (2011). Inflammation in osteoarthritis. Current opinion in rheumatology, 23(5), 471.
[3] Zhang, H., Cai, D., & Bai, X. (2020). Macrophages regulate the progression of osteoarthritis. Osteoarthritis and cartilage, 28(5), 555-561.
[4] Dai, M., Sui, B., Xue, Y., Liu, X., & Sun, J. (2018). Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials, 180, 91-103.
[5] Chang, M. X., & Xiong, F. (2020). Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: recent advances and future directions. Molecules, 25(22), 5342.
[6] Pålsson-McDermott, E. M., & O’Neill, L. A. (2020). Targeting immunometabolism as an anti-inflammatory strategy. Cell research, 30(4), 300-314.