Tweaking the Growth of Stem Cells for Better Therapies
- Their extracellular vesicles change depending on how they are grown.

Researchers publishing in ACS Nano have described how culturing stem cells on nanogratings instead of flat substrates changes the effects of the extracellular vesicles (EVs) they send, potentially paving the way to a new system of therapies.
When signaling is critical
Many of the effects of stem cells can be traced back to the EVs, such as exosomes, that are sent by these cells, rather than the proliferation of the stem cells themselves [1]. Previous work has found that EVs have beneficial effects on muscle regeneration [2]. Therefore, a substantial amount of recent research has focused on cultivating and isolating these intercellular messengers for potential therapeutic use.
However, like any message, the contents of an EV are affected by both the sender and the circumstances under which it was sent. Stem cells send different EVs depending on what they are doing, and differentiating, rather than proliferating, stem cells had improved muscle regeneration in even young mice [3].
Being able to control the behavior of cells is a challenge, but it appears to have a relatively simple solution: topology. Previous work has found it to be possible to change the behavior of stem cells by changing the shape of the substrate on which they are grown [4]. These researchers put these two concepts together, controlling EVs by controlling the behavior of stem cells.
When shape affects biochemistry
Pulling from their previous work, the researchers chose nanogratings used in this study that aligned the stem cells in a way that simulated living tissue [5]. As expected, these cells were considerably more likely to differentiate into functional muscle tissue than their flat-substrate counterparts, and the resulting tissue showed substantially more muscle fibers and tubes. Using nanogratings also significantly upregulated p38 MAPK, a compound responsible for muscular differentiation.
EVs were found to be a large part of these effects. Blocking EVs with two different compounds removed the effects of the nanogratings almost entirely, whether the EVs were blocked on the way in or on the way out.
The EVs of cells cultured on flat surfaces or nanogratings were of similar concentration and size. However, their contents were different: the nanograting-cultured cells had fewer fats and sugars in their EVs (nEVs) than the flat-cultured ones (fEVs). The specific contents of the EVs were not examined, however; unpacking and analyzing these contents will be a problem for future work.
The researchers then drew stem cells from the muscle tissue of aged mice and exposed them to EVs of both kinds. Interestingly, fEVs did improve the formation of muscle tissue somewhat, but not to the extent of nEVs. On the other hand, fEVs were more effective at encouraging these aged cells to proliferate.
Each, in sequence, is needed to work
Using 3D-printed tissue generated from the stem cells of aged mice, the researchers performed many combinations of EV treatment. Neither fEVs nor nEVs by themselves were particularly beneficial. fEVs and nEVs given in combination were also of little help. However, the researchers discovered that, if fEVs were given early on, and then nEVs were delivered later, significant muscle regeneration could be induced in this construct.
The researchers found that this was also true for living animals. Injured mice given fEVs, nEVs, or both at once showed no physical benefit. However, introducing fEVs to induce proliferation, and then nEVs to promote differentiation, substantially improved the muscle regeneration of these animals, bringing them nearly to the levels of young mice.
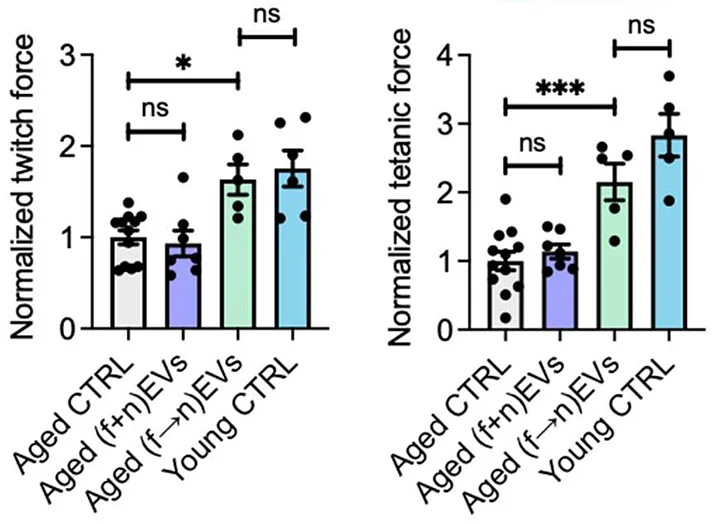
This concept of sequential EV therapy is entirely novel and represents a possible new avenue of treatment for the treatment of the muscle wasting of sarcopenia along with regular muscle injury and sports medicine. However, these are still just mouse experiments so far, and trials will be required to bring this approach to the clinic.
Literature
[1] Wiklander, O. P., Brennan, M. Á., Lötvall, J., Breakefield, X. O., & El Andaloussi, S. (2019). Advances in therapeutic applications of extracellular vesicles. Science translational medicine, 11(492), eaav8521.
[2] Casado-Díaz, A., Quesada-Gómez, J. M., & Dorado, G. (2020). Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Frontiers in Bioengineering and Biotechnology, 8, 146.
[3] Choi, J. S., Yoon, H. I., Lee, K. S., Choi, Y. C., Yang, S. H., Kim, I. S., & Cho, Y. W. (2016). Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. Journal of Controlled Release, 222, 107-115.
[4] Xu, B., Magli, A., Anugrah, Y., Koester, S. J., Perlingeiro, R. C., & Shen, W. (2018). Nanotopography-responsive myotube alignment and orientation as a sensitive phenotypic biomarker for Duchenne Muscular Dystrophy. Biomaterials, 183, 54-66.
[5] Wang, K., Man, K., Liu, J., Meckes, B., & Yang, Y. (2023). Dissecting Physical and Biochemical Effects in Nanotopographical Regulation of Cell Behavior. ACS nano, 17(3), 2124-2133.







