Reversing Senescence in Cells Using Ultrasound
- Mechanical stimuli might work just as well as chemical ones.
A new study claims that low-frequency ultrasound can reverse aspects of replicative and chemically induced senescence in vitro [1].
Not just chemicals
The age-related increase in senescent cell burden is thought to contribute to many processes of aging. Most of the attempts to deal with it involve senolytics: drugs that eliminate senescent cells.
However, it may be possible to re-educate them instead. Senomorphics are compounds that change senescent cells in a way that renders them benign, but they are much less common. The authors of this new pre-print study (it has not yet been peer-reviewed) claim to have found an even more impressive way to solve the senescent cell problem: by rejuvenating them with ultrasound.
Mechanical stimuli may go as far as chemical ones in restoring senescent cells. Connected to each other through the extracellular matrix, cells are constantly reacting to myriads of mechanical signals. Recently, it was found that cells can even be reprogrammed by culturing them on a surface with specific qualities [2].
The sound of rejuvenation
Interestingly, this new study started in a completely different direction. The same team had demonstrated several years earlier that low-frequency ultrasound (LFU) causes apoptosis (cellular death) in cancer cells. This time, the scientists tried to kill senescent cells in a similar way. Surprisingly, when subjected to LFU, senescent cells did the opposite: they started to move and reproduce again.
Cellular senescence is a notoriously heterogeneous phenomenon that looks different in various cell types and can be triggered by many stressors. To test the generality of the effect that they had discovered, the researchers applied LFU to cells in which senescence was induced by one of four compounds: doxorubicin, hydrogen peroxide, sodium butyrate, and bleomycin sulfate.
Most tests were done in bleomycin sulfate-treated cells. The researchers waited for 22 days after the treatment to make sure the cells were indeed senescent. By that time, all cell divisions ceased, and the cells’ migration was low. However, in 48 hours after LFU treatment, over 30% of cells underwent divisions, and cell migration grew twofold.
Scientists also documented the morphology and movement of the mitochondria in the cells, which is a good indicator of cellular health. In the senescent cells, mitochondria were often larger than usual and fused, which is abnormal. Following the treatment, the mitochondria grew smaller and considerably more agile.
The LFU treatment caused a dramatic decrease in several senescence markers. However, the researchers did not observe an increase in apoptosis, suggesting that LFU did not exert a senolytic effect. One of the common features of senescent cells is the senescence-associated secretory phenotype (SASP), a mix of molecules that senescent cells emit, causing inflammation and inducing senescence in neighboring cells. Following the treatment, levels of eight SASP components decreased significantly, including the pro-inflammatory molecules IL-6, TNF-α, IFN-γ, and VEGF. There was also a significant increase in the average telomere length in LFU-treated senescent fibroblasts.
The researchers were able to show that LFU increases autophagy: the process of removing intracellular junk. Adding rapamycin, which promotes autophagy by inhibiting the protein mTOR, amplified the effects of LFU. Interestingly, LFU alone also caused some inhibition of mTOR. Another longevity-related protein that apparently had a part in the process was sirtuin1. Inhibiting it largely blocked the LFU-induced rejuvenation of senescent cells.
Is it like exercise?
Probably the most common cause of senescence is replication. Most somatic cells stop replicating after a certain number of passages (divisions) and start exhibiting signs of senescence. This is what happened to control fibroblasts in this study, starting about passage 15. However, fibroblasts that were treated with LFU after each passage, simply continued to divide, maintaining a healthy growth rate until at least passage 24.
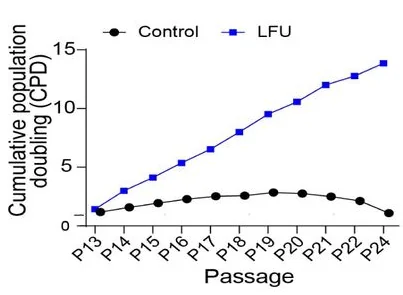
The scientists noted that LFU’s effect on cells has some similarities with that of exercise, another type of mechanistic influence. “We suggest that the effects of LFU mimic many of the effects of exercise at a cellular level with the added benefit that LFU can penetrate the human body to reach internal organs”, they wrote. Exercise has been shown to reduce cellular senescence [3], although its effects might be more of a chemical nature.
These studies show that senescent cells can be mechanically rejuvenated by LFU without transfection or other biochemical manipulations. The ultrasound pressure waves restore normal behavior irrespective of whether senescence is induced by chemical treatment or by repeated replication. There is no apoptosis with LFU and videos of senescent cells before and after LFU show a dramatic increase in cell motility as well as growth.
Literature
[1] Kumar, S., Maroto, R., Powell, S., Margadant, F., Blair, B., Rasmussen, B. B., & Sheetz, M. (2022). Rejuvenating Senescent Cells and Organisms with Only Ultrasound. bioRxiv, 2022-12.
[2] Shou, Y., Teo, X. Y., Wu, K. Z., Bai, B., Kumar, A. R., Low, J., … & Tay, A. (2023). Dynamic Stimulations with Bioengineered Extracellular Matrix‐Mimicking Hydrogels for Mechano Cell Reprogramming and Therapy. Advanced Science, 2300670.
[3] Zhang, X., Englund, D. A., Aversa, Z., Jachim, S. K., White, T. A., & LeBrasseur, N. K. (2022). Exercise counters the age-related accumulation of senescent cells. Exercise and sport sciences reviews, 50(4), 213.








