Utilizing One of the Naked Mole Rat’s Abilities
- Transferring a gene to mice extends their lifespan.

Publishing in Nature, a team of researchers including Vadim Gladyshev, Steve Horvath, and Vera Gorbunova has investigated the role of hyaluronan, which naked mole rats have in abundance, as a protective mechanism in a mouse model.
An established mechanism
Naked mole rats are notably resistant to cancer, and these researchers have previously determined that this resistance is, at least in part, due to high-molecular-mass hyaluronan (HMM-HA) [1]. Hyaluronan is a component of the extracellular matrix that is composed of long repeating chains of glucosamine and glucuronic acid. While smaller forms of hyaluronan (LMM-HA) are associated with inflammation [2] and cancer [3], HMM-HA is anti-inflammatory in nature [4], which may also explain some of the naked mole rat’s longevity.
However, while these benefits had been previously explored, the naked mole rat’s particular tissue-infusing amounts of hyaluronan had not been examined in other species. To that end, these researchers decided to create a mouse model that expresses HMM-HA in the way that the naked mole rat does. They used relatively large samples of mice for this lifespan study, with approximately 80-90 mice in both the treatment and control groups listed in the various results.
Not good for early development, but good for longevity
Because HMM-HA interferes with embryonic development and naturally accumulates in naked mole rats later in life, the researchers controlled its expression, activating it only upon injections of the commonly used molecule tamoxifen. Interestingly, the altered mice only had more HMM-HA in certain tissues when tamoxifen was administered; the muscle tissue contained eight times as much, the heart was unaffected, and the kidneys and intestines contained twice as much. The researchers note that the mice’s levels of hyaluronidase, which breaks down hyaluronan, were not affected and that naked mole rats have much less hyaluronidase.
Even with that being the case, the mice were profoundly affected. Mice naturally get, and die of, cancer. 80% of the old, untreated mice had signs of cancer, but only 40% of the older treated group had such signs. Even when skin cancer was directly induced in the mice through application of a toxin, the HMM-HA mice had significantly fewer tumors.
With no change in overall bodyweight, there were multiple beneficial physical effects. Treated older mice performed better on the commonly used rotarod and forelimb grip strength tests. The treatment group retained stronger bone connectivity, was considerably less frail in old age, and was even biologically younger according to epigenetic testing done on liver tissue. Lifespan, itself, was significantly but slightly improved as a result of the HMM-HA treatment.
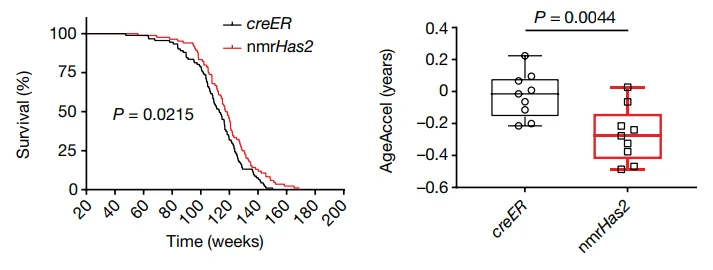
Measurements of gene expression and inflammation backed up these results. The treated group had fewer changes with aging and significantly less inflammation than the control group. The researchers note that these differences do not correspond to any other longevity treatment, suggesting that a combination treatment may be even more effective. Oxidative stress and intestinal health were also positively affected by the additional HMM-HA.
Already in use
Hyaluronan injections are already in use in the clinic for the treatment of arthritis. However, the hyaluronan in those treatments is not being produced by the body’s own cells and suffused throughout the muscles, as it was in these mice. Given the wide-ranging benefits displayed in this study, it may be worthwhile to determine if it is possible and safe to use mRNA or similar technologies to help human beings produce, and retain, more of this apparently life-extending compound.
Literature
[1] Tian, X., Azpurua, J., Hine, C., Vaidya, A., Myakishev-Rempel, M., Ablaeva, J., … & Seluanov, A. (2013). High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature, 499(7458), 346-349.
[2] Cyphert, J. M., Trempus, C. S., & Garantziotis, S. (2015). Size matters: molecular weight specificity of hyaluronan effects in cell biology. International journal of cell biology, 2015.
[3] Zhang, G., Lu, R., Wu, M., Liu, Y., He, Y., Xu, J., … & Gao, F. (2019). Colorectal cancer‐associated ~6kDa hyaluronan serves as a novel biomarker for cancer progression and metastasis. The FEBS Journal, 286(16), 3148-3163.
[4] Muto, J., Yamasaki, K., Taylor, K. R., & Gallo, R. L. (2009). Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Molecular immunology, 47(2-3), 449-456.








