Hyaluronic Acid: Benefits, Side Effects, and Research
Age-related changes lead to a five-fold decrease in the concentration of skin hyaluronic acid content [1]. The effects of this change on appearance from youth to old age are striking. To make matters worse, the reduction in hyaluronic acid is not limited to superficial tissue, nor is it simply a change in appearance. Hyaluronic acid is decreased in the extracellular matrix between every cell with age, and this comes with functional consequences. Evidence suggests that this phenomenon is a major driver of the diseases of middle age and that its decline can short-circuit longevity.
What is hyaluronic acid?
Hyaluronic acid is a linear biopolymer whose synthesis begins in the cell cytoplasm and is completed in the cell membrane. It is synthesized from repeating disaccharide units of N-acetyl-glucosamine and glucuronic acid. At the completion of synthesis, it is released into the extracellular matrix (ECM), where it performs numerous functions in conjunction with other ECM constituents.
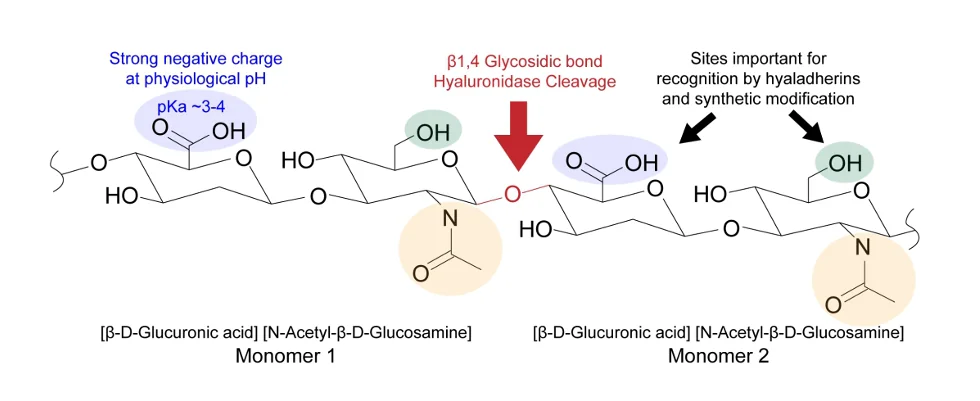
This is hyaluronic acid with reactive sites highlighted. The carboxylic acids, shown in blue, provide a strong negative charge at physiological pH to attract water molecules. Hydroxyl groups, independently present and present as constituents of carboxylic acid groups, attract hyaladherins (special proteins that attach themselves to hyaluronic acid) and provide sites amenable to natural and synthetic modification.
Hyaluronic acid functions as a hydration mechanism, a lubricant, a shock absorber, and a signaling molecule [2]. As a mechanism of hydration, it is crucial for the maintenance of optimal concentrations of water in ECM. At the chemical level, its negatively charged backbone attracts the positive poles of the hydrogen atoms in water.
Therefore, as hyaluronic acid becomes depleted, the ability of the body to maintain optimal hydration is compromised. The bulk of chemical reactions that take place in the body require aqueous media to do so, so dehydration not only leads to significant decrements in physical and mental performance but, if sufficiently severe, may be life-threatening. This is particularly true for the elderly [3-5].
Shock absorption
The ability of hyaluronic acid to hold water is also the basis for its lubricating and shock absorption effects. This is well illustrated by its role in the knee joint. The lubricating fluid in the knee joint (synovial fluid) consists mostly of water and hyaluronic acid. As a linear biopolymer, stacks of waterlogged hyaluronic acid can easily slide past one another inside of the knee, thus acting as a lubricant and enabling smooth joint articulation.
Further, when the knee joint is subjected to acute compressive forces, hyaluronic acid in the joint cavity absorbs the shock, as the force of compression exceeds the intermolecular force holding the water molecules to hyaluronic acid. This results in the lateral dispersion of water molecules, like jumping and landing on a waterfilled sponge. When the compressive force is removed, the attractive force of hyaluronic acid draws water molecules back again to absorb the next acute compressive force.
Without this mechanism, joints would quickly deteriorate, and the depletion of hyaluronic acid greatly hastens the destruction of the knee. In addition to being a major constituent of synovial fluid, hyaluronic acid is also a major constituent of cartilage. When cartilage wears out, a condition referred to as bone-on-bone arthritis can result. Ultimately, this may lead to the need for total knee replacement.
Injecting hyaluronic acid into knees with damaged cartilage can alleviate joint pain and slow the degenerative processes associated with osteoarthritis. Interestingly, it also has the remarkable ability to reduce the sensitivity of neighboring nerves to pain and to aid in their regeneration. Evidence indicates that hyaluronic acid organizes the extracellular matrix, thereby facilitating the migration of the regenerating axons.
Hyaluronic acid with a molecular weight of greater than 40 kilodaltons has been shown to produce a short-term analgesic effect, and raising this to 860 or 2300 produces a much longer analgesic effect. These effects are believed to result from the interaction of hyaluronic acid with its receptors [6,7].
The need for acute force reduction is not limited to the skeletal system. Significant biomechanical forces are generated by the heart and echo through the vascular system with each heartbeat. Hyaluronic acid is integrated into the architecture of the heart and blood vessels, providing wall viscosity, a property that enables the heart and blood vessels to elastically recover energy that would otherwise be lost. This reduces pulse pressures and promotes adequate pulse wave propagation [8,9].
Without hyaluronic acid to dampen these acute biomechanical stressors, significant increases in oxidative damage would likely result. Excessive biomechanical forces are known to trigger various signaling cascades, such as PKCζ, ASK1, JNK, and NADPH oxidase, which can lead to increased production of reactive oxygen and nitrogen species (ROS and NOS, respectively). This leads to changes in gene expression, including NF-κB, AP1, Nox, and BMP4, which trigger the development of abnormalities in cell shape along with apoptosis, cell proliferation, and inflammation [10,11].
Hyaluronic acid signaling
As an extracellular matrix (ECM) component, hyaluronic acid can regulate how cells migrate, divide, differentiate, and die. It interacts with cellular receptors and binds to other ECM components to stage responses to injury, inflammation, and carcinogenesis. Depending on the context, it can produce markedly different effects on inflammation, cell migration, and cell proliferation.
Such diverse effects occur because of modifications to its structure, including changes to polymer size and shape, binding partners, availability, and receptor engagement and signaling. Essentially, hyaluronic acid is a dynamic molecular fabric that is continuously woven, unraveled, decorated with different proteins, and then defaced in accordance with whatever functionality is needed, as it triggers numerous cell surface receptors in different tissues.
These receptors include cluster of differentiation 44 (CD44), Receptor for Hyaluronan Mediated Mobility (RHAMM/CD168), Lymphatic-Vessel Endothelial Hyaluronan Receptor 1 (LYVE1), and other hyaladherins. CD44 comes in several forms that vary in their functions. The interaction between CD44 receptors and hyaluronic acid is affected by its concentration and molecular weight as well as special sugars that alter its functionality. The receptors may also change how they respond if phosphorus is attached to the CD44 receptor (phosphorylation), a common mechanism for altering protein function.
CD44 is also known to react with other ECM proteins, including matrix metalloproteins, growth factors and cytokines [12]. Studies of the CD44 gene indicate that it is primarily involved in T and B cell development, particularly in regulating the earliest stages of T and B cell differentiation and the later stages of T and B cell activation in response to immunological stimuli, such as bacteria, viruses, and cancer cells [12,13].
RHAMM/CD168, the other principal cell surface receptor for HA, mediates cell migration during tissue repair and inflammation and the activation of numerous signaling pathways. These pathways involve Src and other kinase protein complexes of focal adhesions, which are structures that form mechanical links between the skeletons of cells and the ECM. Src is an oncoprotein, which means that it can transform a normal cell into a tumor cell.
An extensive body of research indicates that hyaluronic acid may play a significant role in the development of cancer at all stages. Through RHAMM, hyaluronic acid may trigger Src to activate several receptor tyrosine kinase pathways that promote the survival and proliferation of cancer cells. It can also promote the formation of cancer stem cells that may initiate tumors and resistance to resistance to chemotherapeutic interventions. Different molecular weights have been shown to play different roles in both normal and cancer cell behavior [14].
Hyaluronic acid also interacts with LYVE1, which is like CD44 but is expressed in cells lining the lymphatic system [13]. The lymphatic system drains fluid from tissues, transports fat, and produces lymphocytes (white blood cells) and other immune cells that destroy pathogens
Hyaluronic acid and its derivatives in the clinic
Hyaluronic acid’s vital role in ECM and its other properties has led to its successful deployment in a wide range of medical treatments, including, but not limited to, osteoarthritis [15], cartilage regeneration [15], loss of vitreous fluid from the eyeball [16], dry eye [17], skin damage [2], vascular damage [13], peripheral nerve damage [6,18], soft tissue defects after deep burns [19], surgical reconstruction [20], and cancer therapy [21,22]. It is also used as a drug delivery agent in the treatment of cancer and certain eye disorders [23].
Hyaluronic acid, cancer, and longevity
Cancer and longevity are related phenomena: both are adaptations of early developmental programs. The crucial difference is that cancer is driven by dysregulated growth and development programs and longevity depends upon a finely tuned one. However, the processes that drive cancer and enable longevity often use the same or very similar chemistries. This includes hyaluronic acid, which is necessary for both normal growth and development as well as cancer development.
Cancer cells vary in their relationships to different types of hyaluronic acid, its synthases (HASs), and its receptors [24]. The total volume and type of hyaluronic acid present in tissues is a function of how much of it is being synthesized and how much is being degraded. The degradation process is related to the function of hyaluronidases, which are enzymes that break it down. Additionally, the weight of hyaluronic acid is also governed by these two processes. Hyaluronic acid of different molecular sizes can prompt biological activities that counteract one another [25].
Several potentially troubling findings pertaining to hyaluronic acid’s relationship to cancer have been uncovered. For instance, certain cancer cells, like pancreatic cancer cells, are fueled by hyaluronic acid [26]; others express excessive amounts of hyaluronic acid and its receptors [27,28]. Other studies have shown that hyaluronic acid injection can promote tumor growth [29]. Its production by cancer cells is associated with metastasis in some instances [30]. For this reason, some members of the research community have issued cautionary statements regarding the long-term consumption of oral and injectable hyaluronic acid supplements [31].
The molecular weight of hyaluronic acid has been shown to play a key role in whether it drives or obstructs pathological processes. Hyaluronic acid weight influences inflammation, cellular proliferation, and tissue remodeling. In general, hyaluronic acid with high molecular weight inhibits cellular proliferation and inflammation. Lower-weight hyaluronic acid appears to have the opposite effect [13].
Interestingly, it is known that naked mole rats do not develop cancer and this finding is a direct result of the volume and type of hyaluronic acid they produce [32]. They have been found to produce higher molecular weight hyaluronic acid compounds that are significantly different than those found in mice of comparable size. Evolutionary theorists suspect that the increased molecular weight and volume of hyaluronic acid in naked mole rats evolved as an adaptation to the need to move freely through underground tunnels.
Like humans, naked mole rats use hyaluronic acid throughout their bodies. Therefore, it is particularly enriched in the skin, heart, brain, joints, and kidneys of naked mole rats [32]. They also do not typically develop arthritis and recover from traumatic joint injuries exceptionally quickly [33]. In fact, naked mole rats live about five times longer than other rodents of comparable size [34]. Death in naked mole rats does not appear to be related to age. The most common causes of death among this species results from fighting for the colony queen, predation, starvation, and infection [35].
Hyaluronic acid supplementation side effects
Hyaluronic acid is primarily supplemented via injection, direct application to the skin, and oral supplementation. Injections are normally either given in the skin for cosmetic applications or in the joints for the purposes of pain reduction, joint lubrication, and dissipation of compressive forces that would further damage the joints. A 2018 review of 17 studies looked at single and multiple injections into the knee joints of patients suffering from osteoarthritis. All studies reported an decrease in pain (up to 55% for 25 months), and no adverse effects were reported [36].
Dermal fillers are used to improve appearance but are known to carry certain risks, including swelling, redness, bruising, and pain at the injection site. These side effects are generally transient, but in some cases, there has been persistent swelling, pain, and nodule formation. In these instances, hyaluronidases were injected to break it down [37]. On balance, dermal fillers are considered very safe for the vast majority of patients [38].
Oral supplementation with hyaluronic acid is considered a safe and effective means of improving skin health and joint function [15,39].
Disclaimer
This article is only a very brief summary, is not intended as an exhaustive guide, and is based on the interpretation of research data, which is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you. We do not endorse supplement use or any product or supplement vendor, and all discussion here is for scientific interest.
Literature
[1] E. Papakonstantinou, M. Roth, and G. Karakiulakis, “Hyaluronic acid: A key molecule in skin aging,” Dermatoendocrinol., vol. 4, no. 3, pp. 253–258, Jul. 2012
[2] A. Yasin, Y. Ren, J. Li, Y. Sheng, C. Cao, and K. Zhang, ”Advances in Hyaluronic Acid for Biomedical Applications” Frontiers in Bioengineering and Biotechnology , vol. 10. 2022 910290
[3] D. Benton and H. A. Young, “Do small differences in hydration status affect mood and mental performance?,” Nutr. Rev., vol. 73, no. suppl_2, pp. 83–96, Sep. 2015
[4] S. N. Cheuvront and R. W. Kenefick, “Dehydration: Physiology, Assessment, and Performance Effects,” Comprehensive Physiology. pp. 257–285, Jan. 10, 2014
[5] M. Shimizu et al., “Physical Signs of Dehydration in the Elderly,” Intern. Med., vol. 51, no. 10, pp. 1207–1210, 2012
[6] K.-K. Wang et al., “Hyaluronic acid enhances peripheral nerve regeneration in vivo,” Microsurgery, vol. 18, no. 4, pp. 270–275, Jan. 1998
[7] S. Gotoh et al., “Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats.,” Ann. Rheum. Dis., vol. 52, no. 11, pp. 817 LP – 822, Nov. 1993
[8] M.-N. Gonzalo, F. J. W., G. D. J, H. R. P., and K. J. C., “Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4,” J. Am. Coll. Cardiol., vol. 75, no. 17, pp. 2169–2188, May 2020
[9] Q. Xu, “Biomechanical-stress-induced Signaling and Gene Expression in the Development of Arteriosclerosis,” Trends Cardiovasc. Med., vol. 10, no. 1, pp. 35–41, 2000
[10] E. A. Zemskov et al., “Biomechanical Forces and Oxidative Stress: Implications for Pulmonary Vascular Disease,” Antioxid. Redox Signal., vol. 31, no. 12, pp. 819–842, Jan. 2019
[11] N. Noguchi and H. Jo, “Redox going with vascular shear stress,” Antioxid. Redox Signal., vol. 15, no. 5, pp. 1367–1368, Sep. 2011
[12] D. Vigetti, E. Karousou, M. Viola, S. Deleonibus, G. De Luca, and A. Passi, “Hyaluronan: Biosynthesis and signaling,” Biochim. Biophys. Acta – Gen. Subj., vol. 1840, no. 8, pp. 2452–2459, 2014
[13] G. Abatangelo, V. Vindigni, G. Avruscio, L. Pandis, and P. Brun, “Hyaluronic Acid: Redefining Its Role,” Cells , vol. 9, no. 7. 2020
[14] Y. Sohara et al., “Hyaluronan Activates Cell Motility of v-Src-transformed Cells via Ras-Mitogen–activated Protein Kinase and Phosphoinositide 3-Kinase-Akt in a Tumor-specific Manner,” Mol. Biol. Cell, vol. 12, no. 6, pp. 1859–1868, Jun. 2001
[15] S. Bowman, M. E. Awad, M. W. Hamrick, M. Hunter, and S. Fulzele, “Recent advances in hyaluronic acid based therapy for osteoarthritis,” Clin. Transl. Med., vol. 7, no. 1, p. 6, 2018
[16] S. Yu et al., “Injectable self-crosslinking hydrogels based on hyaluronic acid as vitreous substitutes,” Int. J. Biol. Macromol., vol. 208, pp. 159–171, 2022
[17] J. Pinto-Fraga, A. López-de la Rosa, F. Blázquez Arauzo, R. Urbano Rodríguez, and M. J. González-García, “Efficacy and Safety of 0.2% Hyaluronic Acid in the Management of Dry Eye Disease,” Eye Contact Lens, vol. 43, no. 1, 2017
[18] H. Xu et al., “Sustainable release of nerve growth factor for peripheral nerve regeneration using nerve conduits laden with Bioconjugated hyaluronic acid-chitosan hydrogel,” Compos. Part B Eng., vol. 230, p. 109509, 2022
[19] Y.-W. Ding, Z.-Y. Wang, Z.-W. Ren, X.-W. Zhang, and D.-X. Wei, “Advances in modified hyaluronic acid-based hydrogels for skin wound healing,” Biomater. Sci., vol. 10, no. 13, pp. 3393–3409, 2022
[20] P. Gentile et al., “Use of Platelet Rich Plasma and Hyaluronic Acid in the Treatment of Complications of Achilles Tendon Reconstruction,” World J. Plast. Surg., vol. 5, no. 2, pp. 124–132, May 2016
[21] J. M. Wickens et al., “Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy,” Drug Discov. Today, vol. 22, no. 4, pp. 665–680, 2017
[22] X. Hou et al., “Recent advances in hyaluronic acid-based nanomedicines: Preparation and application in cancer therapy,” Carbohydr. Polym., vol. 292, p. 119662, 2022
[23] X. Zhang, D. Wei, Y. Xu, and Q. Zhu, “Hyaluronic acid in ocular drug delivery,” Carbohydr. Polym., vol. 264, p. 118006, 2021
[24] M. S. Karbownik and J. Z. Nowak, “Hyaluronan: Towards novel anti-cancer therapeutics,” Pharmacol. Reports, vol. 65, no. 5, pp. 1056–1074, 2013
[25] A. G. Tavianatou, I. Caon, M. Franchi, Z. Piperigkou, D. Galesso, and N. K. Karamanos, “Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer,” FEBS J., vol. 286, no. 15, pp. 2883–2908, Aug. 2019
[26] P. K. Kim et al., “Hyaluronic acid fuels pancreatic cancer cell growth,” Elife, vol. 10, p. e62645, 2021.
[27] T. Lee et al., “Minimum hyaluronic acid (HA) modified magnetic nanocrystals with less facilitated cancer migration and drug resistance for targeting CD44 abundant cancer cells by MR imaging,” J. Mater. Chem. B, vol. 5, no. 7, pp. 1400–1407, 2017
[28] H. S. H. et al., “Elevated tissue expression of hyaluronic acid and hyaluronidase validates the HA-HAase urine test for bladder cancer,” J. Urol., vol. 165, no. 6 Part 1, pp. 2068–2074, Jun. 2001
[29] Y. Matsui, M. Inomata, K. Izumi, K. Sonoda, N. Shiraishi, and S. Kitano, “Hyaluronic acid stimulates tumor-cell proliferation at wound sites,” Gastrointest. Endosc., vol. 60, no. 4, pp. 539–543, 2004
[30] K. Kimata, Y. Honma, M. Okayama, K. Oguri, M. Hozumi, and S. Suzuki, “Increased Synthesis of Hyaluronic Acid by Mouse Mammary Carcinoma Cell Variants with High Metastatic Potential,” Cancer Res., vol. 43, no. 3, pp. 1347–1354, Mar. 1983.
[31] P. Simone and M. Alberto, “Caution Should be Used in Long-Term Treatment with Oral Compounds of Hyaluronic Acid in Patients with a History of Cancer,” Clin. Drug Investig., vol. 35, no. 11, pp. 689–692, 2015
[32] X. Tian et al., “High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat,” Nature, vol. 499, no. 7458, pp. 346–349, 2013
[33] T. Taguchi et al., “Naked mole-rats are extremely resistant to post-traumatic osteoarthritis,” Aging Cell, vol. 19, no. 11, p. e13255, Nov. 2020
[34] Y. H. Edrey, M. Hanes, M. Pinto, J. Mele, and R. Buffenstein, “Successful Aging and Sustained Good Health in the Naked Mole Rat: A Long-Lived Mammalian Model for Biogerontology and Biomedical Research,” ILAR J., vol. 52, no. 1, pp. 41–53, Jan. 2011
[35] B. P. Lee, M. Smith, R. Buffenstein, and L. W. Harries, “Negligible senescence in naked mole rats may be a consequence of well-maintained splicing regulation,” GeroScience, vol. 42, no. 2, pp. 633–651, 2020
[36] R. Altman, J. Hackel, F. Niazi, P. Shaw, and M. Nicholls, “Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: A systematic review,” Semin. Arthritis Rheum., vol. 48, no. 2, pp. 168–175, 2018
[37] S. Van Dyke, G. P. Hays, A. E. Caglia, and M. Caglia, “Severe Acute Local Reactions to a Hyaluronic Acid-derived Dermal Filler,” J. Clin. Aesthet. Dermatol., vol. 3, no. 5, pp. 32–35, May 2010
[38] L. C. Becker et al., “Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate,” Int. J. Toxicol., vol. 28, no. 4_suppl, pp. 5–67, Jul. 2009
[39] C. Kawada et al., “Ingested hyaluronan moisturizes dry skin,” Nutr. J., vol. 13, p. 70, Jul. 2014


