What Alkaline Phosphatase Is and Does
The primary function of phosphatases is to remove phosphate groups from proteins, changing how they function and relate to other proteins and providing a crucial function in cellular signaling. Alkaline phosphatases (ALPs) are a group of cellular surface enzymes, so called because they operate at higher, more alkaline pH values. ALP is most active in bone, liver, and intestinal tissue. Normal serum ALP values for adults are between 40-125 IU/L.
ALP types
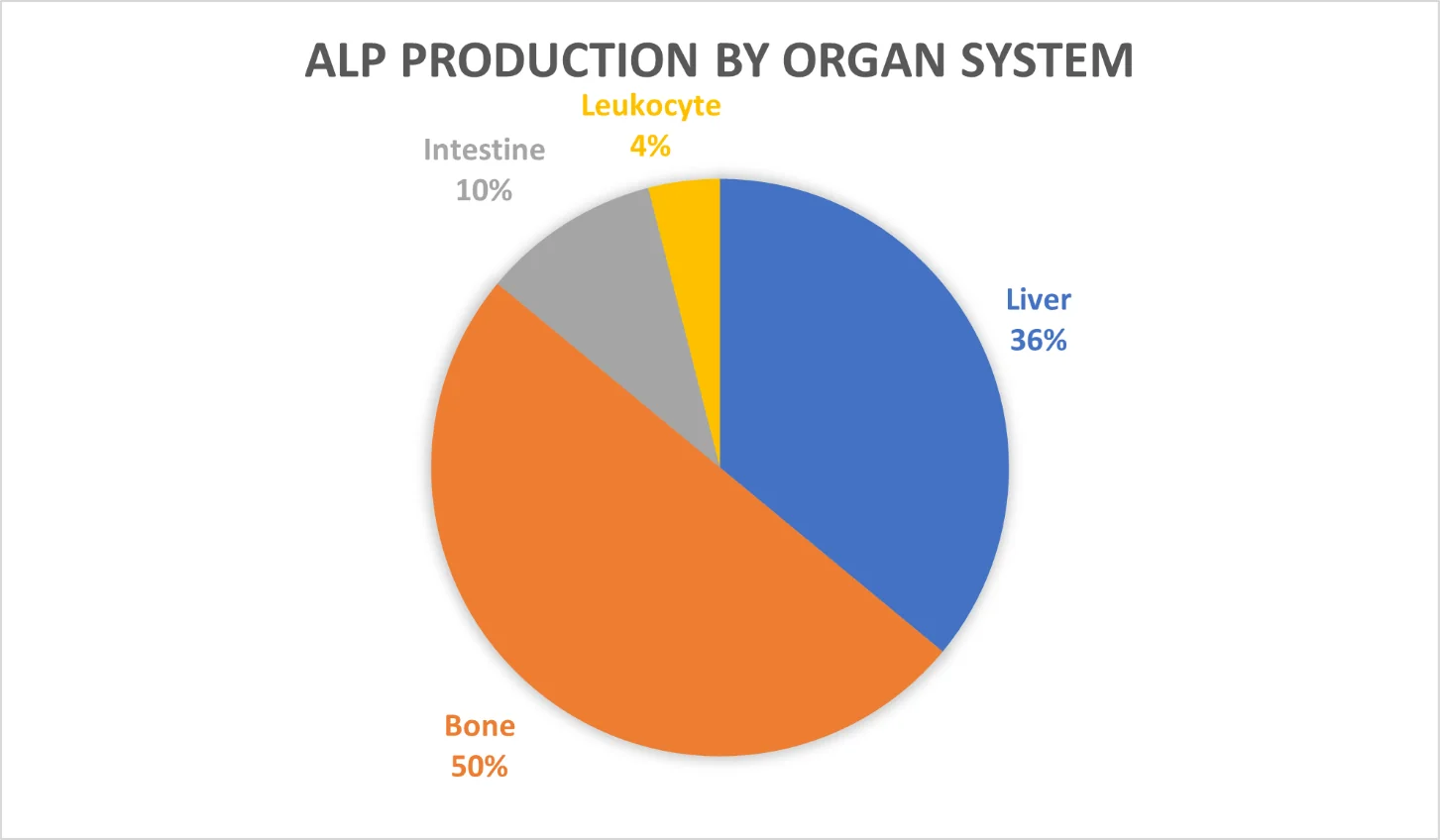
There are six basic ALPs. Alpha-1 ALP, heat-stable alpha-2 ALP, and heat-sensitive alpha-2 ALP are produced in the liver. Pre-beta ALP is produced in bone, gamma ALP is produced in the intestine, and leukocyte ALP is produced in leukocytes [1].
Alpha-1 constitutes 10% of serum ALP. It is made by cells that reside on the surfaces of liver tubes that collect bile, a fluid that aids digestion. Alpha-1 ALP will rise sharply in serum if those tubes are blocked, which can occur due to built-up scar tissue or compression resulting from the growth of a nearby tumor [2].
The heat-sensitive variety of alpha-2 ALP constitutes about 25% of the serum total. This type of ALP is made by liver cells and goes up if there is inflammation in the liver (hepatitis). Hepatitis can be induced by viral, bacterial, traumatic, and alcohol and drug-related causes [1,3]. The heat-stable variety of alpha-2 accounts for 1% of the total ALP, which increases during pregnancy. The absence of an increase during this period suggests the death of the fetus [4].
Pre-beta ALP accounts for 50% of serum ALP. It originates in bone, where it is produced by bone-building cells called osteoblasts. In adulthood, elevation would be considered abnormal. During childhood, however, pre-beta ALP is increased due to its role in calcification, a part of active bone building. Additionally, this type of ALP can be elevated due to certain Illnesses, such as rickets and Paget’s disease. Rickets is strongly associated with vitamin D deficiency, which is known to raise serum ALP [5].
Gamma ALP makes up 10% of total ALP. It is made by intestinal cells and is increased in ulcerative colitis after gastrectomy surgery.
Finally, leukocyte ALP constitutes about 4% of total ALP and originates from leukocytes. It is increased in lymphomas and decreased in chronic myeloid leukemia. It is not found in serum [6].
Sometimes, abnormal ALP isozymes arise. For example, the Regan isoenzyme is an abnormal ALP isoenzyme resembling the placental ALP isozyme. It appears in about 15% of cancer cases in the lung, liver, and gut. Heavy smoking also increases the Regan isoenzyme in the blood [1,7]. Another example of abnormal ALP is the Nagao isoenzyme, which is also present in carcinomas and metastasis [8].
Normal levels of total ALP in adults range from 40 to 125 IU/L. In children, levels are higher (42-362 IU/L) because of the effects of bone growth.
A two- to threefold increase in ALP is commonly seen in liver diseases caused by infection, alcohol, or cancer. Levels of 10 to 12 times the normal upper limit may result from blockage caused by gallstones or pressure in the bile duct by tumors in the head of the pancreas. It can also be caused by intrahepatic cholestasis, which may be due to viruses that cause liver infection or by drugs such as chlorpromazine.
Exceedingly high ALP (10 to 25 times the upper limit) are seen in bone diseases in which the activities of bone-making cells (osteoblasts) are increased. This can occur in Paget’s disease, rickets, osteomalacia, osteoblastoma, metastatic bone cancer, and hyperparathyroidism.
Increasing ALP is associated with all-cause mortality and aging in women
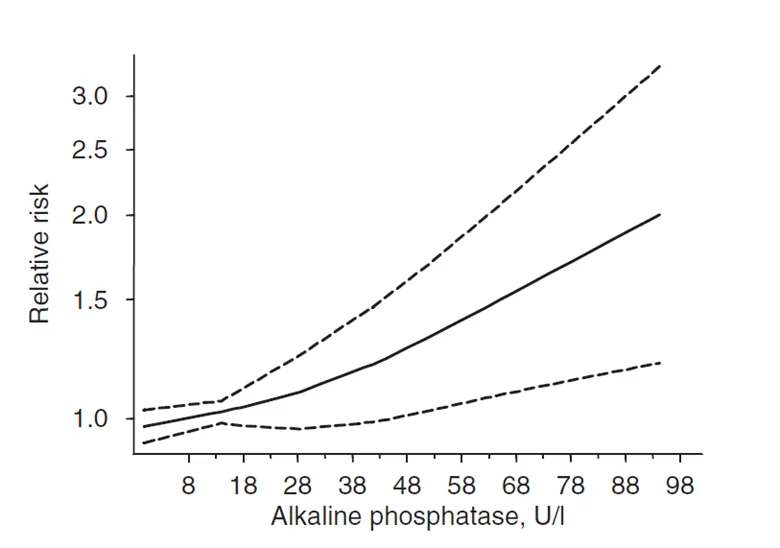
As serum ALP levels rise, so does the relative risk of death, according to two systematic reviews of relevant studies (meta-analyses). In the first review, four studies that included nine million adults found that ALP values greater than 48 IU/L were associated with significantly increased all-cause mortality [9].
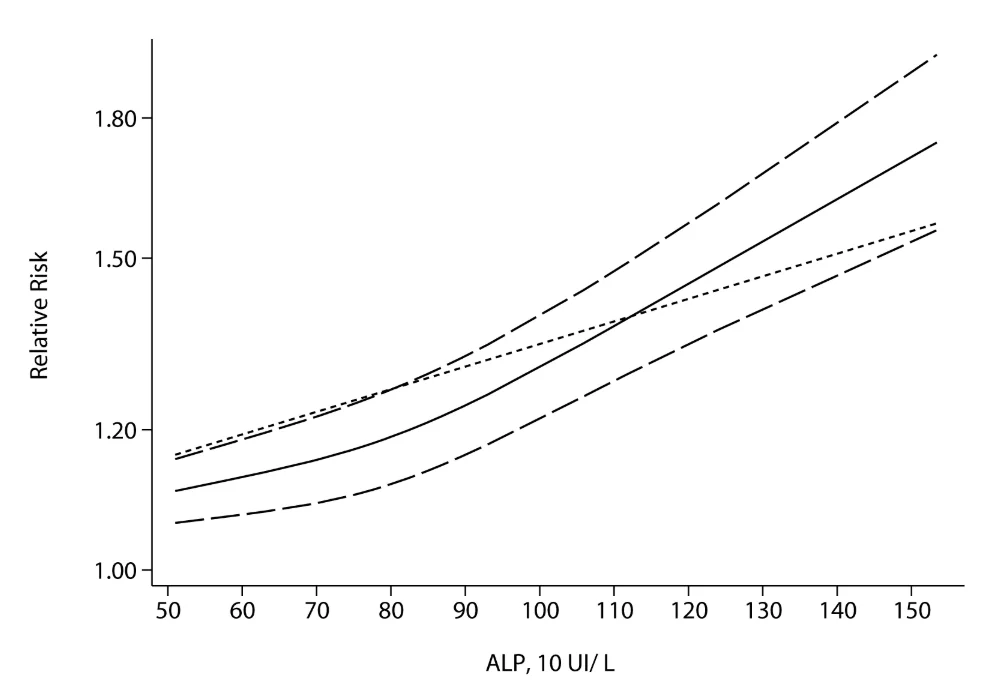
The second meta-analysis included four studies and 147,634 subjects. This study found that an ALP level of 50 IU/L was correlated with the lowest risk of death from all causes [10].
These studies confirm that increasing levels of ALP are associated with greater all-cause mortality, but it is not clear based on published studies how strong the association between high ALP and aging is. A 2015 study looked at serum ALP in 658 individuals. The data did not show a clear relationship between age and ALP in men; however, it did show a clear relationship between age and ALP for women [11].
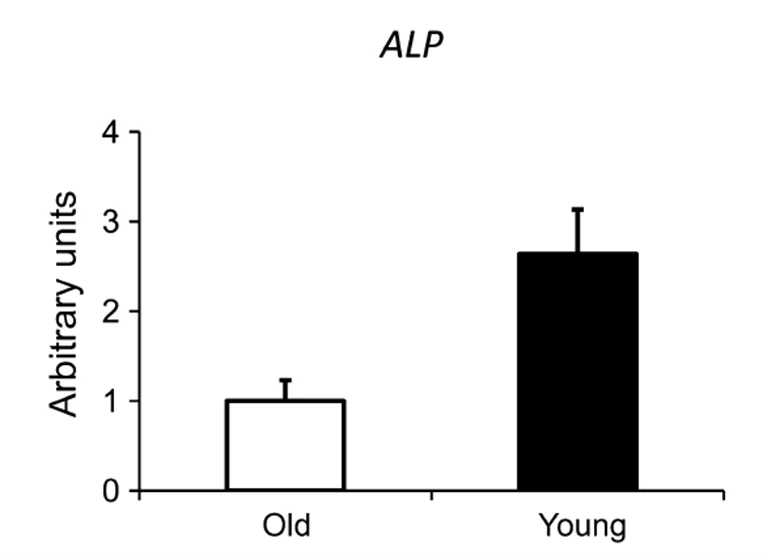
Evidence suggests that sex-based differences in alkaline phosphatase metabolism are related to menopause, which is strongly associated with bone loss in women. A study of 100 women looked at differences in serum calcium and ALP pre- vs. post-menopause. The findings demonstrated that the serum calcium level was significantly lower in the post-menopausal group than in the pre-menopausal group, while the ALP level was slightly higher.
The authors concluded that an increase in bone turnover accelerates bone mass reduction in post-menopausal women, whereas a decrease in bone turnover is associated with the preservation of bone mass [12]. Osteoporosis is associated with higher ALP production in bone [13].
This finding may seem paradoxical given that higher ALP levels in growing children are associated with bone building. However, in this scenario, bone-building osteoblast cells are attempting, unsuccessfully, to keep pace with hyperactive bone-degrading osteoclast cells [13,14]. This mechanism of bone loss despite increased osteoblast activity and increased osteoblast-related ALP activity is also seen in Paget’s disease [15], rickets, and Vitamin D deficiency.
The authors of one study assert that the shift toward bone resorption that begins with the onset of menopause is related to several factors, including decreased estrogen levels and monocyte reprogramming. Monocytes are progenitor cells involved in the immune response as well as bone building and resorption. This study indicates that monocytes are “reprogrammed”, allowing them to “remember” age, menopausal status, and bone formation status in vitro, resulting in more aggressive osteoclasts. This change is thought to be mediated through age-related changes in DNA methylation [16].
Interestingly, a small 2016 study of the relative activity of bone-building and bone-degrading cells found that ALP gene expression in bone was reduced in older populations. However, gene expression related to osteoclast activities were also reduced, but by a smaller margin [17]. This study didn’t look at post-menopausal women. Nonetheless, an additional possibility is that serum ALP increases due to changes in liver and/or intestine function, which are also related to post-menopausal hormone changes and general changes in health as well.
Age-related changes in ALP driven by intestinal and liver ALP fractions
Serum ALP is third in relative importance on Aging.ai’s blood biomarker platform for age analysis. There is a definite increase in serum ALP in general in women with advancing age, and studies suggest that this is related to genetic and endocrine changes that occur after menopause. The modest or minor changes in serum ALP in men do not address potentially significant changes that occur where the ALP in serum is being produced. In other words, pronounced changes in liver, gut, and bone ALP can offset each other but nevertheless indicate pathology.
One area where significant changes in ALP fractions occur in both sexes with age is at the gut-liver axis. Age-related changes in gut microbiota influence nutrient absorption, energy homeostasis, and control of body weight. Changes in the gut microbiome are strongly associated with increased intestinal permeability (leakiness) [18].
An intestinal defense mechanism
Intestinal alkaline phosphatase (IAP) increases in response to gut injury and increased permeability, and ulcerative colitis has been shown to increase levels of IAP. Ulcerative colitis is an inflammatory bowel disease that compromises intestinal barrier function. Similarly, age-associated increases in gut permeability also compromise barrier function and promote endotoxemia, the entry of bacterial toxins, such as lipopolysaccharide, into circulation.
This results in low-grade sterile inflammation that contributes to obesity, metabolic syndrome, and liver injury, including non-alcoholic hepatic steatosis (fatty liver) aka NAFLD [18,19]. Metabolic syndrome and NAFLD are related and have similar prevalence. Metabolic syndrome affects one in three adults [20]. NAFLD is the most common cause of liver disease in Westernized countries, and it affects up to 33% of the general population and 75% of obese patients [21,22].
ALP levels may double in the liver disease hepatic steatosis, particularly as the disease progresses [23]. This is due to blockage of the biliary ducts of the liver where ALP is produced.
IAP is necessary for the prevention of metabolic syndrome and certain kinds of liver disease. IAP is an important brush border enzyme produced in the intestine that is expressed throughout the gastrointestinal tract and secreted into both the intestinal lumen and the bloodstream.
IAP is upregulated as a defensive measure by the body. It defends the body against endotoxemia by dephosphorylation of proinflammatory molecules including lipopolysaccharide (LPS), flagellin, and adenosine triphosphate (ATP) [24]. In the process, LPS-induced immune system-dependent inflammation is reduced, optimal distribution of microbiome populations is maintained, inflammatory bowel disease may be neutralized, and reduction of systemic inflammation ensues [25].
This is a two-way battle, however, because endotoxins promote inflammation and certain pro-inflammatory cytokines such as IL-1β and TNF-α inhibit expression of IAP [26]. If endotoxemia is not quickly checked by IAP, alkaline phosphatase production of the intestine and liver will be altered [25,27].
Marginal increases in serum ALP may not reflect pathological changes in ALP fractions in the liver and intestine. Age-associated changes in gut permeability upregulate ALP activity as a defense mechanism against toxins that may be introduced into the circulatory system. These toxins are associated with systemic inflammation, metabolic syndrome, obesity, and liver disease, which is associated with higher production of liver ALP.
Nutritional approaches to improving gut health can reduce serum ALP
Age-related increases in gut permeability drive inflammation. This further damages the gut and liver while promoting systemic inflammation. Dietary changes that reduce gut permeability and improve gut immune defense are theoretically sound methods of reducing serum ALP. Paradoxically this involves increasing IAP. Studies suggest that this is because IAP directly detoxifies endotoxins like LPS to reduce inflammation. Furthermore, IAP has been shown to improve barrier function by several other mechanisms, including pH calibration, improving the function of tight junctions, and enhancing the population of probiotic bacteria in the colon [28].
ALP is best viewed as a defense mechanism in the context of gut health. There is a difference between lowering ALP by directly inhibiting its production and lowering ALP by eliminating ALP-mobilizing factors such as LPS. For example, one study found that omega-3 fatty acids, commonly found in fish oil and taken as supplements, reduced intestinal inflammation but increased rates of sepsis-induced mortality. The authors concluded that omega-3 fatty acids depressed gut IAP activity and associated LPS detoxification, thereby increasing serum levels of LPS [29].
Nutrients shown to affect IAP
Calcium
Animal studies have shown that IAP can be stimulated by free calcium, bound phosphorus, and vitamins. Supplemental calcium has been shown to reduce intestinal inflammation if administered in the presence of bound phosphorus [30]. Bound phosphorus, also known as inorganic phosphorus, is typically bound to metals such as calcium and iron.
This suggests that free phosphorus, which is often found in dairy, red meat, poultry, seafood, and legumes, might discourage calcium absorption. Phosphorus has been shown to have an inhibitory effect on IAP [31]. This effect has not been associated with any other potentially positive outcomes, such as reduced endotoxin load or decreased intestinal permeability.
K1 and K2
Vitamins K1 and K2 are traditionally associated with blood clotting and bone development. K1 and K2 increase IAP through mechanisms that are not clear [32]. In addition, K2 stimulates osteoblast differentiation and bone ALP [33]. In this context, the increase in ALP is not associated with pathology.
Further, recent discoveries suggest that vitamin K2 prevents arterial calcification (hardening of the arteries) and plays an essential role in the trafficking of calcium into bone matrix [33]. Excessive calcium supplementation, especially in older individuals, accelerates arterial calcification [34,35]. Therefore, anyone considering supplementary calcium to optimize ALP would be wise to also consult a physician about Vitamin K2 supplements.
Vitamin D
Vitamin D3, the bioactive form of vitamin D, regulates the expression of IAP and deficiencies of Vitamin D3 result in inadequate production of IAP. D3 is also necessary to absorb calcium from the gut, which is necessary to maintain bone density. Maintaining adequate intestinal ALP can, paradoxically, keep age-related increases in serum ALP in check by protecting the liver from enterotoxins that would drive up liver ALP. Vitamin K2 is especially important for older women who take vitamin D and calcium supplements [5].
Berberine and metformin
Metformin and berberine inhibit the normal increase in lipopolysaccharide (LPS) that occurs in serum after a high-fat meal. A decrease in LPS suggests that either the composition of gut microbiota has changed or that the intestinal barrier has been modified in a way that blocks normal access by LPS into the bloodstream [36]. This reduces the need to maintain high IAP levels.
Metformin and berberine have also been shown to positively alter the microbiome in animal studies by increasing SCFA-producing bacteria. Similar findings occurred in studies of Type 2 diabetics who take metformin [36]. Metformin increases the abundance of Akkermansia muciniphila [37], which is negatively correlated with obesity, diabetes, and cardiovascular disease [38]. Additionally, it helps to reduce the migration of LPS into the bloodstream and would reduce the need for upregulation of IAP [38].
Probiotics, fiber, and short chain fatty acids
Bacteria such as Ruminococcus and Faecalibacterium, which account for 5-10% of the total microbiome [39,40], digest fiber and use it to produce short-chain fatty acids such as butyrate. Butyrate participates in local and global signaling networks to produce a range of effects, including enhancement of intestinal barrier function, mucosal immunity, and intestinal homeostasis. In turn, these effects can lead to improvements in energy metabolism, promotion of weight loss, reduction of inflammation, and proper functioning of the gut-brain axis, which serves as a communication hub between intestinal bacteria and the brain [41].
The body attempts to raise IAP in the presence of inflammation to detoxify enterotoxins such as LPS and flagellin. The rise in IAP may be offset by certain inflammatory factors that have been shown to depress IAP production. The net effect is an inadequate rise in IAP, which results in incomplete detoxification of enterotoxins, which then pass through the gut wall and wreak havoc on the liver [42], where liver ALP can begin rising to cause an overall increase in serum ALP [43].
Gamma IAP
IAP has been safely administered to humans, and a human recombinant form of IAP has been developed [24]. One study found that oral supplementation with gamma ALP reduces frailty and extends lifespan [44]. Furthermore, supplementation with this variety of ALP is associated with preserved intestinal homeostasis during aging. In fruit flies, gamma ALP supplementation was shown to mitigate gut barrier dysfunction, adverse gut microbiome changes, and endotoxemia, which results from the release of toxic compounds from gut bacteria into circulation. The researchers concluded that oral supplementation of gamma ALP could counteract gut-mediated systemic inflammation, which leads to frailty and other age-related conditions [45].
Potentially negative nutritional factors
Very high-protein diets
High-protein diets, in murine models, have been shown to alter the gut microbiome, damage kidneys, and increase intestinal permeability and systemic inflammation [46]. To date, this has not been demonstrated in human beings, and the high-protein diet used in this study contained 52% protein. While there is no unequivocal definition of a high-protein diet, many dieticians define it as when at least 35% of daily calories come from protein [47]. This mouse diet continued substantially more. This would be atypical for most athletes other than bodybuilders or people who follow carnivorous diets.
High-fat diets
LPS provokes systemic inflammation and exerts growth-promoting effects on fat tissue. High fat intake increases the passage of LPS across the intestinal barrier [48]. IAP limits the effects of LPS in two ways. First, IAP limits the rate at which fatty acids are transported from the gut and thereby limits the entry of LPS into circulation; second, IAP dephosphorylates LPB, removing the bulk of its toxic potential.
Therefore, it is not surprising that several studies have found supplemental IAP can prevent metabolic syndrome. Further studies show that mice without the IAP gene develop fatty liver, high triglycerides, and other symptoms of metabolic syndrome when fed a high-fat diet. Long-chain fatty acids tend to cause a greater rise in intestinal alkaline phosphatase than medium-chain triglycerides do [49].
Fish oil
Several studies have looked at the potential use of omega-3 fatty acids in the treatment of gut disorders. Unfortunately, these studies suggest that while omega-3 fatty acids reduce colonic inflammation, this occurs at the expense of disabling IAP from dephosphorylation LPS [29]. Consequently, mortality was increased among test animals used in this study.
Fish oil as a supplement rose to fame following the publication of Dean Ornish’s publication of Reversing Heart Disease in which it was reported that heart attacks among Native American Intuits was extraordinarily low. Later, it was found that Inuits have the highest rates of hemorrhagic stroke in the world [9,50]. Nonetheless, fish oil and eating fish high in Omega-3 are recommended by the American Heart Association as a means for improving blood lipids and lowering cardiovascular mortality. This suggests that fish oil supplementation should be a context-dependent and individual choice that is not necessarily appropriate for everyone.
Phosphorus
In a study to determine the role of ALP in phosphorus transport, researchers found that low-phosphorus diets caused a compensatory increase in intestinal alkaline phosphatase. This finding suggests that IAP may be increased to improve phosphorus intake. In this context, low phosphorus could potentially distract IAP from its detoxification role [31]. Interestingly, organic phosphorus in some studies has been shown to bind to calcium, potentially undermining its ability to reduce intestinal inflammation [51].
Studies on caloric restriction, ALP, and intestinal permeability
A six-month study following individuals on an American Heart Association diet found two groups had significant decreases in serum alkaline phosphatase and liver lipid content: those who restricted calorie intake by 25% and lost 10% of their body weight and those who followed a liquid diet and lost 15% of their body weight. Alkaline phosphatase decreased by 9% and 10%, respectively. Other biomarkers mechanistically associated with intestinal ALP also improved, including insulin sensitivity, body fat percentage, and serum C-reactive protein [52].
A rodent study looked at the effects of aging and caloric restriction on intestinal permeability, which is associated with IAP researchers found lifetime caloric restriction had no effect on intestinal permeability [53].
Literature
[1] V. Indumati, Integrated Textbook of Biochemistry, 1st ed. Paras Medical Publishers, 2021.
[2] M. Viot, C. Joulin, P. Cambon, B. P. Krebs, M. Schneider, and C. M. Lalanne, “The value of serum alkaline phosphatase alpha 1 isoenzyme in the diagnosis of liver metastases. Preliminary results,” Biomedicine, vol. 31, no. 3, pp. 74–77, 1979, [Online].
[3] N. H. Korner, “Distribution of alkaline phosphatase in serum protein fractions” J. Clin. Pathol., vol. 15, no. 3, pp. 195 LP – 199, May 1962
[4] J. C. Robinson, J. E. Pierce, and B. S. Blumberg, “The serum alkaline phosphatase of pregnancy,” Am. J. Obstet. Gynecol., vol. 94, no. 4, pp. 559–565, 1966
[5] M. Sahay and R. Sahay, “Rickets-vitamin D deficiency and dependency,” Indian J. Endocrinol. Metab., vol. 16, no. 2, pp. 164–176, Mar. 2012
[6] D. B. Okun and K. R. Tanaka, “Leukocyte alkaline phosphatase,” Am. J. Hematol., vol. 4, no. 3, pp. 293–299, 1978
[7] Y. Jiang, X. Li, and D. R. Walt, “Single-Molecule Analysis Determines Isozymes of Human Alkaline Phosphatase in Serum,” Angew. Chemie Int. Ed., vol. 59, no. 41, pp. 18010–18015, Oct. 2020
[8] J. L. Millán and T. Manes, “Seminoma-derived Nagao isozyme is encoded by a germ-cell alkaline phosphatase gene.,” Proc. Natl. Acad. Sci., vol. 85, no. 9, pp. 3024–3028, May 1988
[9] S. K. Kunutsor, T. A. Apekey, D. Seddoh, and J. Walley, “Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis,” Int. J. Epidemiol., vol. 43, no. 1, pp. 187–201, Feb. 2014
[10] J. W. Li, C. Xu, Y. Fan, Y. Wang, and Y. Bin Xiao, “Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis,” PLoS One, vol. 9, no. 7, pp. 1–14, 2014
[11] J. M. Cyphert, C. S. Trempus, and S. Garantziotis, “Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology,” Int. J. Cell Biol., vol. 2015, p. 563818, 2015
[12] T. Bhattarai, K. Bhattacharya, P. Chaudhuri, and P. Sengupta, “Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women,” Malays. J. Med. Sci., vol. 21, no. 1, pp. 58–61, Jan. 2014
[13] Z. W. Lim and W.-L. Chen, “Exploring the association of Bone Alkaline Phosphatases And Hearing Loss,” Sci. Rep., vol. 10, no. 1, p. 4006, 2020
[14] A. M. J. Møller et al., “Aging and menopause reprogram osteoclast precursors for aggressive bone resorption,” Bone Res., vol. 8, no. 1, p. 27, 2020
[15] G. D. Roodman and J. J. Windle, “Paget disease of bone,” J. Clin. Invest., vol. 115, no. 2, pp. 200–208, Feb. 2005
[16] M. Soltan, M. D. Rohrer, and H. S. Prasad, “Monocytes: Super Cells for Bone Regeneration,” Implant Dent., vol. 21, no. 1, 2012
[17] M. Becerikli et al., “Age-dependent alterations in osteoblast and osteoclast activity in human cancellous bone,” J. Cell. Mol. Med., vol. 21, no. 11, pp. 2773–2781, Nov. 2017
[18] L. Drozdowski and A. B. R. Thomson, “Aging and the intestine,” World J. Gastroenterol., vol. 12, no. 47, pp. 7578–7584, Dec. 2006
[19] T. H. Frazier, J. K. DiBaise, and C. J. McClain, “Gut Microbiota, Intestinal Permeability, Obesity-Induced Inflammation, and Liver Injury,” J. Parenter. Enter. Nutr., vol. 35, no. 5S, pp. 14S-20S, Sep. 2011
[20] NIH, “Metabolic Syndrome – What Is Metabolic Syndrome? | NHLBI, NIH.” [Online]. Available: https://www.nhlbi.nih.gov/health/metabolic-syndrome
[21] R. M. Williamson et al., “Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People With Type 2 Diabetes: the Edinburgh Type 2 Diabetes Study,” Diabetes Care, vol. 34, no. 5, pp. 1139–1144, Apr. 2011
[22] M. W. Pantsari and S. A. Harrison, “Nonalcoholic Fatty Liver Disease Presenting With an Isolated Elevated Alkaline Phosphatase,” J. Clin. Gastroenterol., vol. 40, no. 7, 2006
[23] H. L. Brensilver and M. M. Kaplan, “Significance of Elevated Liver Alkaline Phosphatase in Serum,” Gastroenterology, vol. 68, no. 6, pp. 1556–1562, 1975
[24] J. Bilski et al., “The Role of Intestinal Alkaline Phosphatase in Inflammatory Disorders of Gastrointestinal Tract,” Mediators Inflamm., vol. 2017, p. 9074601, 2017
[25] J.-P. Lallès, “Intestinal alkaline phosphatase: novel functions and protective effects,” Nutr. Rev., vol. 72, no. 2, pp. 82–94, Feb. 2014
[26] M. S. Malo, S. Biswas, M. A. Abedrapo, L. Yeh, A. Chen, and R. A. Hodin, “The Pro-inflammatory Cytokines, IL-1β and TNF-α, Inhibit Intestinal Alkaline Phosphatase Gene Expression,” DNA Cell Biol., vol. 25, no. 12, pp. 684–695, Dec. 2006
[27] K. Kaliannan et al., “Intestinal alkaline phosphatase prevents metabolic syndrome in mice,” Proc. Natl. Acad. Sci., vol. 110, no. 17, pp. 7003–7008, Apr. 2013
[28] G. M. Santos et al., “Intestinal Alkaline Phosphatase: A Review of This Enzyme Role in the Intestinal Barrier Function,” Microorganisms, vol. 10, no. 4. 2022.
[29] S. Ghosh et al., “Fish Oil Attenuates Omega-6 Polyunsaturated Fatty Acid-Induced Dysbiosis and Infectious Colitis but Impairs LPS Dephosphorylation Activity Causing Sepsis,” PLoS One, vol. 8, no. 2, p. e55468, Feb. 2013
[30] M. A. A. Schepens, S. J. M. ten Bruggencate, A. J. Schonewille, R.-J. M. Brummer, R. van der Meer, and I. M. J. Bovee-Oudenhoven, “The protective effect of supplemental calcium on colonic permeability depends on a calcium phosphate-induced increase in luminal buffering capacity,” Br. J. Nutr., vol. 107, no. 7, pp. 950–956, 2012
[31] S. A. Kempson, J. K. Kim, T. E. Northrup, F. G. Knox, and T. P. Dousa, “Alkaline phosphatase in adaptation to low dietary phosphate intake.,” Am. J. Physiol. Metab., vol. 237, no. 5, p. E465, Nov. 1979
[32] N. Sogabe, R. Maruyama, T. Hosai, and M. Goseki-Sone, “Enhancement Effects of Vitamin K1 (Phylloquinone) or Vitamin K2 (Menaquinone-4) on Intestinal Alkaline Phosphatase Activity in Rats,” J. Nutr. Sci. Vitaminol. (Tokyo)., vol. 53, no. 3, pp. 219–224, 2007
[33] S. Akbari and A. A. Rasouli-Ghahroudi, “Vitamin K and Bone Metabolism: A Review of the Latest Evidence in Preclinical Studies,” Biomed Res. Int., vol. 2018, p. 4629383, Jun. 2018
[34] J. J. B. Anderson and P. J. Klemmer, “Risk of high dietary calcium for arterial calcification in older adults,” Nutrients, vol. 5, no. 10, pp. 3964–3974, Sep. 2013
[35] R. Flore et al., “Something more to say about calcium homeostasis: the role of vitamin K2 in vascular calcification and osteoporosis,” Eur. Rev. Med. Pharmacol. Sci., vol. 17, pp. 2433–2440, Sep. 2013.
[36] Q. Zhang and N. Hu, “Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus,” Diabetes. Metab. Syndr. Obes., vol. 13, pp. 5003–5014, Dec. 2020
[37] X. Zhang et al., “Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance,” PLoS One, vol. 8, no. 8, 2013
[38] P. D. Cani and W. M. de Vos, “Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila,” Front. Microbiol., vol. 8, p. 1765, Sep. 2017
[39] Z. Mokhtari, D. L. Gibson, and A. Hekmatdoost, “Nonalcoholic Fatty Liver Disease, the Gut Microbiome, and Diet,” Adv. Nutr., vol. 8, no. 2, pp. 240–252, Mar. 2017
[40] J. Serpa et al., “Butyrate-rich Colonic Microenvironment Is a Relevant Selection Factor for Metabolically Adapted Tumor Cells,” J. Biol. Chem., vol. 285, no. 50, pp. 39211–39223, 2010
[41] H. Liu et al., “Butyrate: A Double-Edged Sword for Health?,” Adv. Nutr., vol. 9, no. 1, pp. 21–29, Jan. 2018
[42] J.-B. Soares, P. Pimentel-Nunes, R. Roncon-Albuquerque, and A. Leite-Moreira, “The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases,” Hepatol. Int., vol. 4, no. 4, pp. 659–672, 2010
[43] S. B. Rosalki and N. i Mcintyre, “Biochemical investigations in the management of liver disease,” Oxford Textbook. Clin. Hepatol. Ed, vol. 2, pp. 506–507, 1999.
[44] J. W. Larrick and A. R. Mendelsohn, “Supplementation with Brush Border Enzyme Alkaline Phosphatase Slows Aging,” Rejuvenation Res., vol. 23, no. 2, pp. 171–175, Apr. 2020
[45] F. Kühn et al., “Intestinal alkaline phosphatase targets the gut barrier to prevent aging,” JCI insight, vol. 5, no. 6, p. e134049, Mar. 2020
[46] M. Snelson et al., “Long Term High Protein Diet Feeding Alters the Microbiome and Increases Intestinal Permeability, Systemic Inflammation and Kidney Injury in Mice,” Mol. Nutr. Food Res., vol. 65, no. 8, p. 2000851, Apr. 2021
[47] K. Todd, “High-Protein Diets and Weight Loss – Today’s Dietitian Magazine.”
[48] P. Cani and N. Delzenne, “The role of the gut microbiota in energy metabolism and metabolic disease,” Curr. Pharm. Des., vol. 15, no. 13, pp. 1546–1558, 2009, doi: 10.2174/138161209788168164.
[49] A. P. Day, M. D. Feher, R. Chopra, and P. D. Mayne, “Triglyceride Fatty Acid Chain Length Influences the Post Prandial Rise in Serum Intestinal Alkaline Phosphatase Activity,” Ann. Clin. Biochem., vol. 29, no. 3, pp. 287–291, May 1992
[50] R. D. Horner, G. M. Day, A. P. Lanier, E. M. Provost, R. D. Hamel, and B. A. Trimble, “Stroke mortality among Alaska Native people,” Am. J. Public Health, vol. 99, no. 11, pp. 1996–2000, Nov. 2009
[51] S. Sasaki et al., “A Role of Intestinal Alkaline Phosphatase 3 (Akp3) in Inorganic Phosphate Homeostasis,” Kidney Blood Press. Res., vol. 43, no. 5, pp. 1409–1424, 2018
[52] D. E. Larson-Meyer et al., “Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function,” Obesity (Silver Spring)., vol. 16, no. 6, pp. 1355–1362, Jun. 2008, doi: 10.1038/oby.2008.201.
[53] T. Y. Ma, D. Hollander, V. Dadufalza, and P. Krugliak, “Effect of aging and caloric restriction on intestinal permeability,” Exp. Gerontol., vol. 27, no. 3, pp. 321–333, 1992