A Protective Mechanism Against AMD
- A response to DNA damage seems to help cells resist the disease.

A paper published in Aging explains the relationship of long noncoding RNAs, which change with aging, to age-related macular degeneration (AMD).
A disease of deposits and aging
This paper begins with a discussion of AMD and its prevalence. AMD is the most common cause of vision loss in people over 70 years old [1]. Its main characteristic is the formation of drusen, yellow-brown deposits within the eye. These deposits have been found to contain a wide variety of proteins associated with the complement pathway, a feature of the immune system. They even include amyloid beta, the same protein associated with Alzheimer’s disease [2].
AMD has been shown to be caused by the aging of retinal pigmental epithelial (RPE) cells, and it is possible to model AMD by inducing senescence in these cells [3] or by inducing oxidative stress through sodium iodate. Interestingly, quercetin has been reported to be effective against that stress in a model [4].
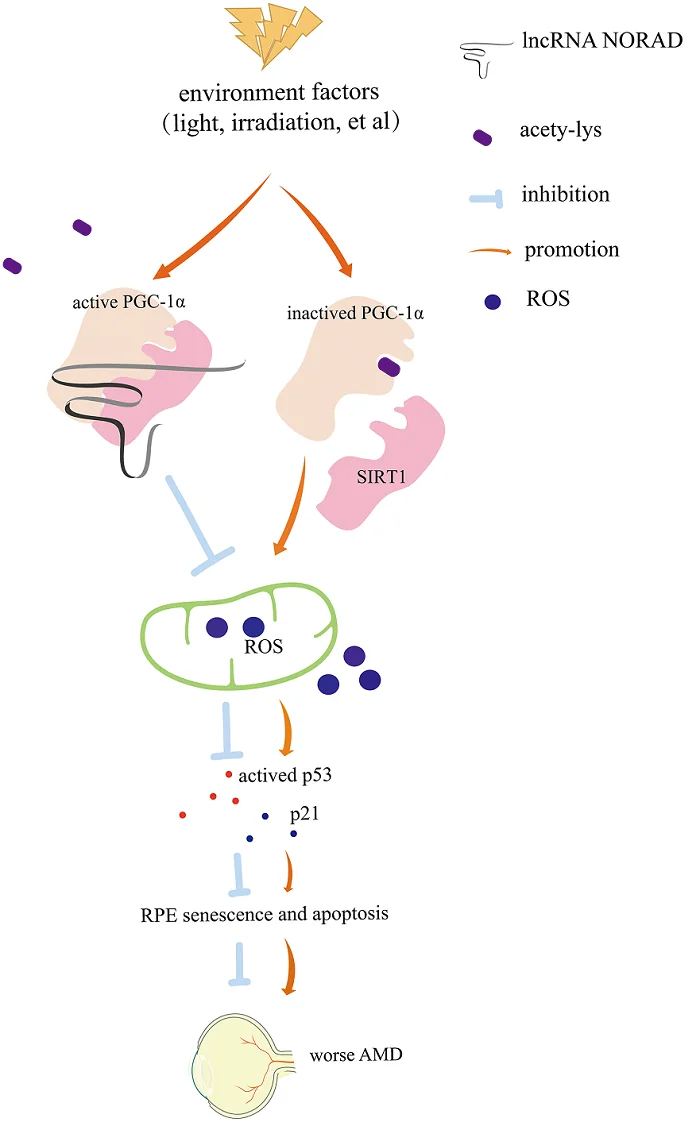
While most RNA strands are meant to execute code stored in DNA, long noncoding RNAs (lncRNAs) are different. Despite their lack of transcription ability, these molecules are associated with multiple aspects of aging. Their relationship to AMD has been previously, but lightly, explored [5]. One group of these lncRNAs, called NORAD, is triggered by DNA damage. NORAD sequesters two key proteins in order to maintain genomic stability [6], and mice without NORAD age more rapidly [7].
The value of NORAD
For the first part of this study, the researchers induced senescence in RPE cells by subjecting them to irradiation. Then, they created a different group of NORAD-deficient RPE cells and subjected them to the same irradiation. As expected, the lack of DNA protection from NORAD drove these cells senescent more quickly, increasing known aging biomarkers along with many of the secreted proteins associated with the drusen of AMD.
The researchers then turned to a mouse model. Along with a control group, NORAD-deficient mice were injected with sodium iodate in the tail vein. As expected, the NORAD-deficient mice suffered more damage, lost more function, and had more age-related proteins concentrated in the eyes, even though there was no significant difference before the sodium iodate injection.
This loss of function was matched by mitochondrial tests. After sodium iodate, reactive oxygen species were considerably more prevalent in the NORAD-deficient mice than their unaltered counterparts. The researchers found that this was true for the NORAD-deficient cells in their culture as well.
Finally, the researchers examined the relationship of NORAD to the well-known sirtuin SIRT1 and the metabolic regulator PGC-1α, whose acetylation is increased in AMD. They found that knocking out NORAD had similar effects to knocking out SIRT1 and that NORAD facilitates the binding of SIRT1 to PGC-1α, which may explain some of its AMD-related effects.
A potential new target
The researchers hold that their mouse and cellular models are very similar to human AMD in the clinic. While the researchers would have liked to overexpress NORAD in order to determine if that would have protective effects against AMD, they were unable to, as overexpressing NORAD caused cellular disruption. The researchers also note that NORAD has different effects on different organs.
However, if a future treatment can increase the amount of NORAD in aging eyes, it may be possible to directly affect a key cause of AMD at its root. Further studies will need to be conducted to determine if such a treatment can be viable.
Literature
[1] Wong, W. L., Su, X., Li, X., Cheung, C. M. G., Klein, R., Cheng, C. Y., & Wong, T. Y. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health, 2(2), e106-e116.
[2] Ohno-Matsui, K. (2011). Parallel findings in age-related macular degeneration and Alzheimer’s disease. Progress in retinal and eye research, 30(4), 217-238.
[3] Chae, J. B., Jang, H., Son, C., Park, C. W., Choi, H., Jin, S., … & Chung, H. (2021). Targeting senescent retinal pigment epithelial cells facilitates retinal regeneration in mouse models of age-related macular degeneration. Geroscience, 43, 2809-2833.
[4] Chang, Y. Y., Lee, Y. J., Hsu, M. Y., Wang, M., Tsou, S. C., Chen, C. C., … & Lin, H. W. (2021). Protective effect of quercetin on sodium iodate-induced retinal apoptosis through the reactive oxygen species-mediated mitochondrion-dependent pathway. International Journal of Molecular Sciences, 22(8), 4056. [5] Blasiak, J., Hyttinen, J. M., Szczepanska, J., Pawlowska, E., & Kaarniranta, K. (2021). Potential of long non-Coding RNAs in age-related macular degeneration. International Journal of Molecular Sciences, 22(17), 9178.
[6] Munschauer, M., Nguyen, C. T., Sirokman, K., Hartigan, C. R., Hogstrom, L., Engreitz, J. M., … & Lander, E. S. (2018). The NORAD lncRNA assembles a topoisomerase complex critical for genome stability. Nature, 561(7721), 132-136.
[7] Kopp, F., Elguindy, M. M., Yalvac, M. E., Zhang, H., Chen, B., Gillett, F. A., … & Mendell, J. T. (2019). PUMILIO hyperactivity drives premature aging of Norad-deficient mice. Elife, 8, e42650.