Bacteria May Be Fueling Cancer with Methionine
- This cancer promotes the growth of these bacteria.

Scientists have found that the tumor microenvironment in lung adenocarcinoma favors methionine-producing bacteria, which, in turn, help the cancer survive nutrient scarcity [1].
Micro-friends or micro-foes?
Our bodies host a mind-bending number of microorganisms, but most effects of this cohabitation are not well understood. Scientists have unearthed links between oral and gut microbiota and aging [2], but what about bacteria residing in other tissues?
In this new study, the researchers deliver a fascinating theory about the role that bacteria might play in lung cancer. It has been known for a while that the tumor microenvironment (TME) can have an altered microbiome [3]. However, how this contributes to cancer development is still mostly a mystery.
Methionine pathways enriched
The researchers first confirmed using human samples that the TME in lung adenocarcinoma (LUAD), one of the deadliest cancers, indeed hosts an altered microbiome compared to adjacent healthy tissue. These changes were unrelated to any infection.
Then, the researchers performed analysis of the bacterial RNA to determine which pathways were differentially expressed in tumor-associated and normal microbiomes. The one that stood out was the methionine production pathway, which is highly enriched in TME samples.
Methionine, associated with cell growth and proliferation [4], is one of the nine essential amino acids, the ones that we cannot produce ourselves and must get from food. Bacteria, however, can make methionine. Interestingly, methionine restriction has been shown to increase healthspan and lifespan in various animal models, including mice [5].
Cancer-driven selection
Cancer cells are constantly dividing, but this uncontrolled growth also creates a hypoxic and nutrient-deficient tumor microenvironment due to insufficient vascularization [6]. The researchers hypothesized that this nutrient deprivation could exert selective pressure on tumor microbiota, favoring methionine-producing bacteria.
They tested their theory in an ingenious experiment, co-culturing LUAD cells with either wild-type E. coli bacteria or genetic mutants stripped of their ability to produce methionine. In a nutrient-rich medium, both types of bacteria grew normally, but in the medium that imitated nutrient-deficient TME conditions, the methionine-producing wild type strain began to dominate. This effect was weaker without LUAD cells present, suggesting that those cells actively contributed to the selective pressure.
The researchers then injected mice with LUAD cells and put the animals on either a methionine-abundant or a methionine-restricted diet. In the latter group, the cancer progressed at a much slower pace, and the viability and motility of the cancer cells were impaired.
In vitro experiments with two LUAD cell strains showed greatly reduced cell viability in lower methionine concentrations:
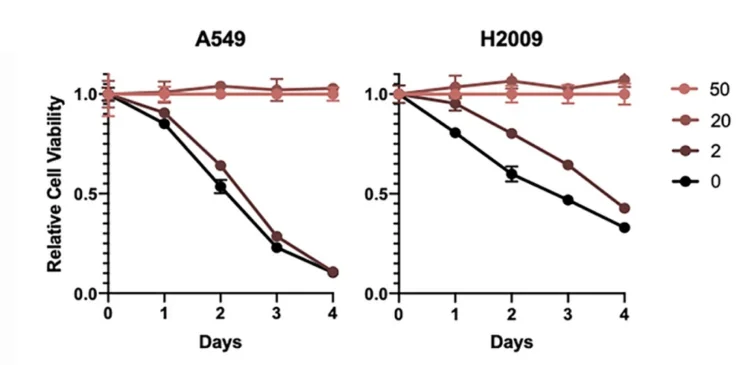
Nutrient-starved LUAD cells could be rescued by adding either methionine or methionine-producing bacteria to the culture. Conversely, adding methionine-deficient bacteria did not improve LUAD cell viability.
Cells can compensate for nutrient deprivation by overexpressing nutrient transporters, including the methionine transporter LAT1. Analyzing samples from two large cohorts of LUAD patients, the researchers found that LAT1 was indeed significantly overexpressed in tumor tissues as opposed to adjacent non-malignant tissues. Moreover, levels of LAT1 expression were negatively correlated with patients’ survival.
Bacteria-cancer nutrient exchange
Finally, the researchers wanted to prove that cells and bacteria can indeed exchange nutrients. They did so by feeding either the bacteria or the cancer cells glucose that contained carbon-14, a radioactive carbon isotope. Then, cells and bacteria were co-cultured, and the researchers were able to detect bacteria-produced biomolecules in cells and vice versa. This crosstalk between cells and bacteria is likely to involve various types of molecules and calls for further investigation.
In conclusion, we have presented data to support a dynamic interaction between tumor and tumor-resident bacterial cells. Specifically, our data supports that the LUAD TME exerts a tumor-specific microbial selection pressure on microbial communities that can in turn contribute to tumor metabolism through the production of methionine at the tumor site. Further, an association between the expression of a methionine transporter with patient survival, suggests therapeutic potential directed towards the bacterial component of the TME. Moreover, our findings indicate a possible crosstalk between the tumor and the surrounding microbiome that may rescue tumor survival under nutrient-deprived states. As nutrient-deprived phenotypes are common among solid tumors and the metabolic potential of the microbiome is broad, this work has wide-reaching implications for tumor behaviour across tissue types including a wide variety of tumor niches.
While scientists have made great progress against blood cancers, solid tumor cancers have proved to be much tougher adversaries. As methionine deprivation has a restricting effect on glioblastoma [7], nutrient deprivation might be their weak spot. If the researchers’ assumption is correct, tumors can potentially be starved by targeting their bacterial accomplices.
Literature
[1] Vega, A. A., Marshall, E. A., Noonan, A. J., Filho, F. S. L., Yang, J., Stewart, G. L., … & Lam, W. L. (2023). Methionine-producing tumor micro (be) environment fuels growth of solid tumors. Cellular Oncology, 1-15.
[2] Ghosh, T. S., Shanahan, F., & O’Toole, P. W. (2022). The gut microbiome as a modulator of healthy ageing. Nature Reviews Gastroenterology & Hepatology, 19(9), 565-584.
[3] Poore, G. D., Kopylova, E., Zhu, Q., Carpenter, C., Fraraccio, S., Wandro, S., … & Knight, R. (2020). Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature, 579(7800), 567-574.
[4] Lauinger, L., & Kaiser, P. (2021). Sensing and signaling of methionine metabolism. Metabolites, 11(2), 83.
[5] Lee, B. C., Kaya, A., & Gladyshev, V. N. (2016). Methionine restriction and life-span control. Annals of the New York Academy of Sciences, 1363(1), 116-124.
[6] Wek, R. C., & Staschke, K. A. (2010). How do tumours adapt to nutrient stress?. The EMBO journal, 29(12), 1946-1947.
[7] Mazor, K. M., Dong, L., Mao, Y., Swanda, R. V., Qian, S. B., & Stipanuk, M. H. (2018). Effects of single amino acid deficiency on mRNA translation are markedly different for methionine versus leucine. Scientific reports, 8(1), 1-13.








