Modulating Macrophages to Prevent Disc Degeneration

A paper published in Aging has shown that it is possible to treat spinal disc degeneration through altering the epigenetics of macrophages.
Macrophages and inflammaging
Macrophages are the clean-up crew of the immune system, engulfing and destroying harmful germs, but their polarization is a double-edged sword. On one hand, M1 macrophages serve on the front line against infection and invasion by foreign matter, spurring inflammation in order to summon additional immune cells and deal with immediate threats. Macrophages that are polarized towards any of the M2 subtypes focus on healing and repair, reducing inflammation and spurring healing processes.
Under normal circumstances, inflammation is a temporary response to a temporary problem. However, when the balance of M1 and M2 macrophages is imbalanced towards M1, inflammation becomes chronic, and this is part of inflammaging, the systemic inflammation that leads to multiple age-related diseases.
Changing macrophages to treat the spine
Lumbar disc degeneration (LDD), which leads to intense pain and crippling disability, is one of the many diseases caused by immune dysregulation. The researchers of this new paper cite prior work showing that macrophages are the only immune cells that infiltrate into the nucleus pulposus [1], which makes up the shock absorbers of the spinal column, and that dysregulated macrophages cause a decline in the extracellular matrix, which also contributes to this disease [2]. However, M2 macrophages are not entirely positive in this regard; the M2c subtype of macrophages produces transforming growth factor beta 1 (TGFβ1), which has a negative effect on LDD.
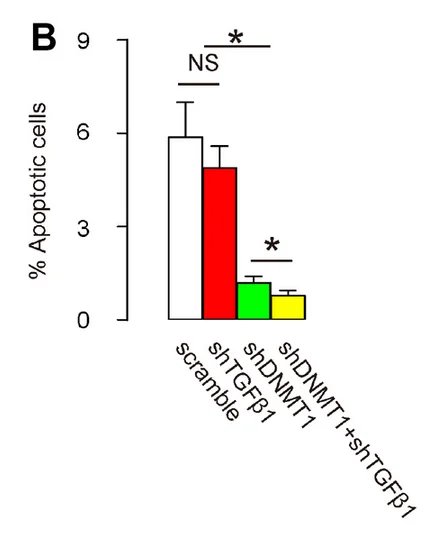
Therefore, in order to treat this disease, the researchers used one adeno-associated virus (AAV) to deplete DNA methyltransferase 1 (DNMT1) from macrophages, as this depletion has been shown to encourage them to polarize towards M2 [3]. The team used another AAV to inhibit TGFβ1.
The results in this mouse model were stark. As the researchers explain, cellular suicide through apoptosis is the central pathology of LDD, and apoptosis was decreased dramatically through suppression of DNMT1 and further decreased through suppressing TGFβ1.
Abstract
Inflammation plays an essential role in the development of lumbar disc degeneration (LDD), although the exact effects of macrophage subtypes on LDD remain unclear. Based on previous studies, we hypothesized that M2-polarization of local macrophages and simultaneous suppression of their production of fibrotic transforming growth factor beta 1 (TGFβ1) could inhibit progression of LDD. Thus, we applied an orthotopic injection of adeno-associated virus (AAV) carrying shRNA for DNA Methyltransferase 1 (DNMT1) and/or shRNA for TGFβ1 under a macrophage-specific CD68 promoter to specifically target local macrophages in a mouse model for LDD. We found that shDNMT1 significantly reduced levels of the pro-inflammatory cytokines TNFα, IL-1β and IL-6, significantly increased levels of the anti-inflammatory cytokines IL-4 and IL-10, significantly increased M2 macrophage polarization, significantly reduced cell apoptosis in the disc degeneration zone and significantly reduced LDD-associated pain. The anti-apoptotic and anti-pain effects were further strengthened by co-application of shTGFβ1. Together, these data suggest that M2 polarization of macrophages induced by both epigenetic modulation and suppressed production and release of TGFβ1 from polarized M2 macrophages, may have a demonstrable therapeutic effect on LDD.
Conclusion
Disc degeneration in the spine is commonly thought of as a wear-and-tear process, something that simply results from the mechanical effects of living life. However, this research shows that, instead, it is influenced by a well-known inflammatory process and that we might possibly be able to do something about it.
Of course, the potential of this line of research is not limited to disc degeneration. Many other age-related conditions are caused by inflammatory processes, and having AAVs in our toolkit against them, encouraging our macrophages to fight back against inflammation instead of spreading it, is very likely to lead to future treatments that directly affect this critical aspect of aging.
Literature
[1] Yang C, Cao P, Gao Y, Wu M, Lin Y, Tian Y, Yuan W. Differential expression of p38 MAPK α, β, γ, δ isoforms in nucleus pulposus modulates macrophage polarization in intervertebral disc degeneration. Sci Rep. 2016; 6:22182.
[2] Koike Y, Uzuki M, Kokubun S, Sawai T. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003; 28:1928–33.
[3] Wang, X., Cao, Q., Yu, L., Shi, H., Xue, B., & Shi, H. (2016). Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI insight, 1(19).








