NAD+ Enters Cells via Newly Discovered Pathway
- Surprise as Researchers discover NMN can enter the cell without becoming NR first

A new study published in Nature Metabolism finally reveals the answer to how NMN enters the cell in order to become NAD+ and that it does not need to be converted into NR to do so.
In the last few years, there has been considerable interest in restoring levels of the nicotinamide adenine dinucleotide (NAD+) coenzyme to combat age-related diseases. Evidence suggests that NAD+ systemically declines with age in a variety of organisms, including rodents and humans, which contributes to the development of many age-related diseases and metabolic conditions.
What is NAD+?
Nicotinamide adenine dinucleotide (NAD) is a coenzyme found in all living cells. It is a dinucleotide, which means that it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base, and the other contains nicotinamide.
NAD facilitates redox reactions, carrying electrons from one reaction to another. This means that NAD is found in two forms in the cell; NAD+ is an oxidizing agent that takes electrons from other molecules in order to become its reduced form, NADH. NADH can then become a reducing agent that donates the electrons it carries. The transfer of electrons is one of the main functions of NAD, though it also performs other cellular processes, including acting as a substrate for enzymes that add or remove chemical groups from proteins in post-translational modifications.
In metabolism, NAD+ helps facilitate cellular functions, DNA repair, growth, and many more things. Quite simply, without NAD+, life would not be possible.
NAD+ is created from simple building blocks, such as the amino acid tryptophan, and it is created in a more complex way via the intake of food that contains nicotinic acid (niacin) or other NAD+ precursors. These different pathways ultimately feed into a salvage pathway, which recycles them back into the active NAD+ form.
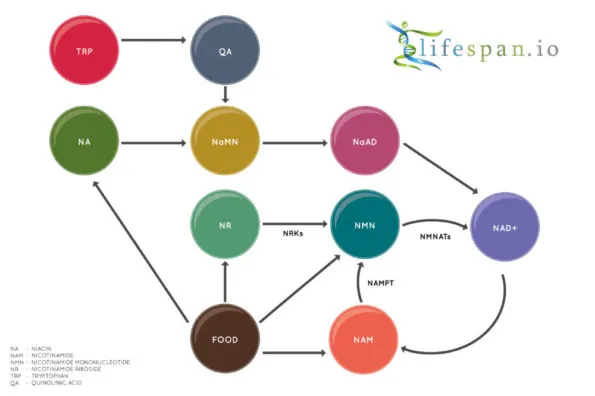
Nicotinamide adenine dinucleotide (NAD+) biology has seen a great deal of interest in the last few years, partially due to the discovery of two precursors of NAD+ biosynthesis, nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), which both increase NAD+ in the cells of multiple tissues.
However, how NMN enters the cell has been a highly debated topic and some researchers had claimed that it cannot and some had suggested that it can via then-unknown transporters.
NMN appears to enter the cell via a newly discovered transporter
A new study published in Nature Metabolism finally reveals the answer to how NMN enters the cell in order to become NAD+ [1]. The researchers in this new study successfully identify the transporter for NMN, which helps to solve the mystery of how mammals absorb and produce NAD+.
Abstract
Nicotinamide mononucleotide (NMN) is a biosynthetic precursor of nicotinamide adenine dinucleotide (NAD+) known to promote cellular NAD+ production and counteract age-associated pathologies associated with a decline in tissue NAD+ levels. How NMN is taken up into cells has not been entirely clear. Here we show that the Slc12a8 gene encodes a specific NMN transporter. We find that Slc12a8 is highly expressed and regulated by NAD+ in the mouse small intestine. Slc12a8 knockdown abrogates the uptake of NMN in vitro and in vivo. We further show that Slc12a8 specifically transports NMN, but not nicotinamide riboside, and that NMN transport depends on the presence of sodium ion. Slc12a8 deficiency significantly decreases NAD+ levels in the jejunum and ileum, which is associated with reduced NMN uptake as traced by doubly labelled isotopic NMN. Finally, we observe that Slc12a8 expression is upregulated in the aged mouse ileum, which contributes to the maintenance of ileal NAD+ levels. Our work identifies a specific NMN transporter and demonstrates that Slc12a8 has a critical role in regulating intestinal NAD+ metabolism.
Prior to this new discovery, it was generally thought that NMN could not enter the cell directly and that it had to convert back into NR in order to do so. This was via a dephosphorylation step taking place on the surface of cells, which converts NMN back into NR prior to entering the cell through equilibrative nucleotide transporters before finally changing back into NMN by being re-phosphorylated by NR kinases.
The new study reveals that a previously characterized membrane protein called Slc12a8 is also an NMN transporter. The Slc12a8 transporter has a variety of unique properties, including requiring sodium, not chloride, to transport NMN. It is also specific to NMN and does not even transport the chemically similar nicotinic acid mononucleotide (NaMN) which is almost the same structurally.
This newly discovered method of NMN transport does not mean that the uptake of NMN via dephosphorylation does not happen or that it is still not important in metabolism. However, it does show that the transport of NAD+ precursors does occur via an alternative and previously unknown mechanism, which builds on our current knowledge of NAD+ biology considerably.
There is also evidence that this mechanism is more prevalent in certain kinds of tissues compared to others, as Slc12a8 expression is around 100-fold times higher in the small intestine of mice than it is in the brain or fat tissue. This suggests that different cells, tissues, and organs may use the various NAD+ precursors in differing amounts and types.
Also of note is that the expression of Slc12a8 actually increases in the gut of aged mice, while NAD+ levels begin to decline. This suggests that this increase of Slc12a8 expression is a compensatory mechanism to try to maintain metabolic homeostasis and resist this aspect of aging.
Remarkably, the function of the Slc12a8 NMN transporter becomes crucial in aged individuals compared with young ones. In response to significant decreases in NAD+ levels, the aged ileum upregulates Slc12a8 expression and tries to maintain its NAD+ levels. When enough NMN is supplied, this feedback system can function adequately to maintain levels of NAD+ comparable to those in young ilea. Therefore, increasing NMN availability or stimulating the function of the NMN transporter could effectively counteract age-associated NAD+ decline in the aged small intestine.
Finally, the authors of this new study speculate that Slc12a8 expression in the gut also facilitates the uptake of NMN, NR, and NAD+ from naturally occurring food sources, including fruits, vegetables, and also milk [2-3]. However, the amount of NMN required to increase NAD+ in a mouse or human is far beyond the amount found in dietary sources. NMN is found in various foods but at concentrations of less than 1 mg per kg; the dose required to raise NAD+ is hundreds of milligrams.
Our cells can also produce NAD+ through the de novo pathway, which begins with the most basic building block, the amino acid tryptophan (Trp), so it is not clear that dietary NMN can impact NAD+ levels in a significant way or if Slc12a8 expression affects NAD+ levels beyond the liver, where NMN is mostly metabolized.
The authors suggest that it is plausible that the gut microbiome may produce NMN similarly to how it produces short-chain fatty acids such as butyrate, which it produces through breaking down plant matter and fiber.
Another possibility is that certain gut bacterial species may produce NMN. If this is the case, the Slc12a8 NMN transporter would likely play a critical role in the symbiotic regulation of NAD+ biosynthesis between the microbiota and the host.
Further studies will need to be conducted to see if there are populations of bacteria producing NMN in this manner, though it is a distinct possibility that they might be. The gut microbiome is a fascinating area of research and is potentially linked to aging in ways such as inflammaging.
Conclusion
This new study is an interesting development in the NAD+ story and will no doubt fuel further heated debate, especially among people who want to establish their NAD+ precursors as the superior choice for a dietary supplement.
The easiest way to settle the matter would be to run proper comparative studies with compounds such as NR, NMN, and niacin. This is something within easy reach of our community, as studies could be run free from the conflicts of interest that surround studies conducted or funded by the manufacturers of these compounds. Only then can we start to separate the scientific facts from the desire to make money, which is always a concern when studies are funded by the manufacturers of such compounds.
Meanwhile, our knowledge of NAD+ biology continues to grow, and, no doubt, more surprises are in store down the road as the NAD+ story continues to evolve. It will be interesting to see if these authors’ hypothesis about the gut microbiome pans out.
Literature
[1] Grozio, A. et al. N at. Metab. https://doi.org/10.1038/s42255-018-0009-4 (2018).
[2] Trammell, S. A., Yu, L., Redpath, P., Migaud, M. E., & Brenner, C. (2016). Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk–3. The Journal of nutrition, 146(5), 957-963.
[3] Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., … & Yoshino, J. (2016). Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell metabolism, 24(6), 795-806.








