Alpha-Lipoic Acid: Benefits, Side Effects, and Research
Alpha-lipoic acid (ALA) is a popular supplement and is often marketed as an antioxidant supplement. It serves multiple functions in the human body, and it may be useful against certain conditions.
What is alpha-lipoic acid?
ALA was first discovered in 1937. It began when Esmond Snell found that some bacteria relied on potato juice to reproduce [1]. This led to it becoming known as a potato growth factor.
Its first isolation happened in 1951 by Lester Reed [2]. In 1959, German scientists used it to treat liver failure caused by death-cap mushroom poisoning [3].
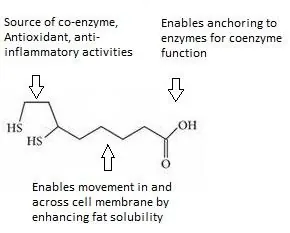 ALA functions as an essential metabolic coenzyme, antioxidant, and anti-inflammatory [4]. ALA is a medium-chain fatty acid with a carboxylate on one end, which anchors onto proteins to perform as a co-enzyme. The other side consists of a ring structure that harbors two sulfur atoms, which give ALA most of its reactive properties. The main part of ALA consists of a medium-length carbon chain.
ALA functions as an essential metabolic coenzyme, antioxidant, and anti-inflammatory [4]. ALA is a medium-chain fatty acid with a carboxylate on one end, which anchors onto proteins to perform as a co-enzyme. The other side consists of a ring structure that harbors two sulfur atoms, which give ALA most of its reactive properties. The main part of ALA consists of a medium-length carbon chain.
This combination allows ALA to stop lipid peroxidation reactions, which are a type of free radical oxidation [5, 6]. Its structure is unusual compared to other antioxidants, which are typically either water- or fat-soluble.
Sources of alpha-lipoic acid
ALA is internally produced by some animals, but humans only produce it in small amounts. There are a number of foods that contain ALA:
- Peas
- Potatoes
- Brussels sprouts
- Broccoli
- Sweet potatoes
- Red meat
- Rice bran
- Spinach
- Tomatoes
- Yams
- Yeast
- Organ meats
It is often sold as a dietary supplement and marketed as an antioxidant. The amount of ALA in supplements is significantly higher than would normally be obtained in the diet.
Alpha-lipoic acid as a co-enzyme
ALA is a non-protein co-enzyme that is often bound to numerous protein-based multienzyme complexes, including the glycine cleavage system and four α-ketoacid dehydrogenase complexes [7].
The glycine cleavage system helps make nucleic acids. These include DNA, RNA, ATP, ribosomes, and other important molecules for life. The four α-ketoacid dehydrogenase complexes are part of the Krebs cycle, which turns energy from carbohydrates, fats, and proteins into carbon dioxide and the chemical energy that cells use: adenosine triphosphate (ATP).
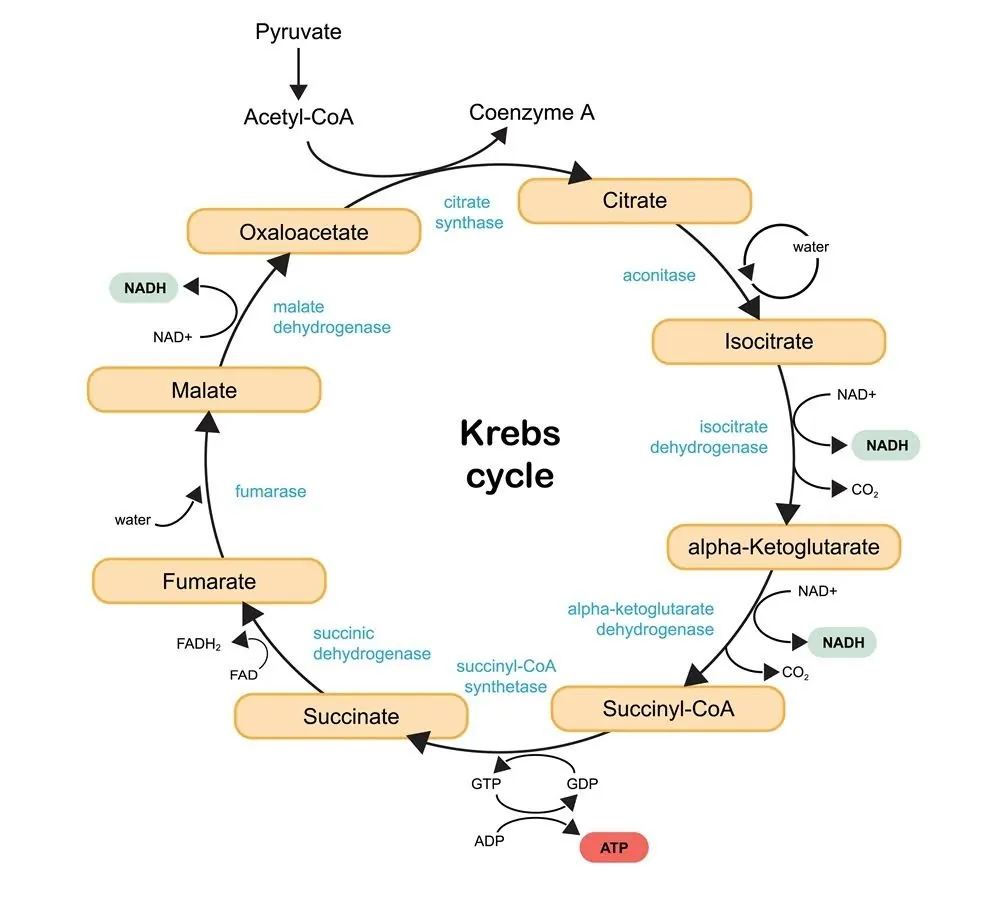
The Krebs cycle also creates many amino acid precursors and the reducing agent NADH, which provides electrons to the electron transport chain. This process helps produce ATP [8].
Alpha-lipoic acid’s role in the antioxidant defense system
Exogenous ALA enters cells through the sodium-dependent multivitamin transporter (SDVT). This is a protein that helps move vitamins and other important substances like biotin and pantothenic acid [9].
Upon entry into the watery interior of the cell, ALA is reduced by one of several thioredoxin-fold proteins. Reduced ALA is customarily referred to as DHLA. ALA’s antioxidant potential is greatest in this form [10].
ALA interacts with other biomolecules in ways that counter the development of age-related diseases and conditions. These conditions include metabolic syndrome [11, 12], diabetes [4], high blood pressure, atherosclerosis [13], ischemia-reperfusion injury [13], cancer [14, 15], and various neurodegenerative disorders [16].
Alpha-lipoic acid and metabolic syndrome
Metabolic syndrome manifests as a constellation of symptoms that include hypertension, insulin resistance, diabetes, obesity, and rising levels of cholesterol and triglycerides.
ALA supplements appear to reduce blood sugar. They may also reduce obesity, lower blood pressure, and normalize cholesterol and triglyceride levels [12]. ALA likely works as a coenzyme in energy production, acts as an antioxidant, and has anti-inflammatory properties.
ALA’s most profound effects on metabolic syndrome may be mediated through its effects on adenosine monophosphate kinase (AMPK), an enzyme that regulates metabolism based on the ratio of AMP and ATP [11, 17]. The difference between AMP and ATP is the two phosphate molecules on the tail:
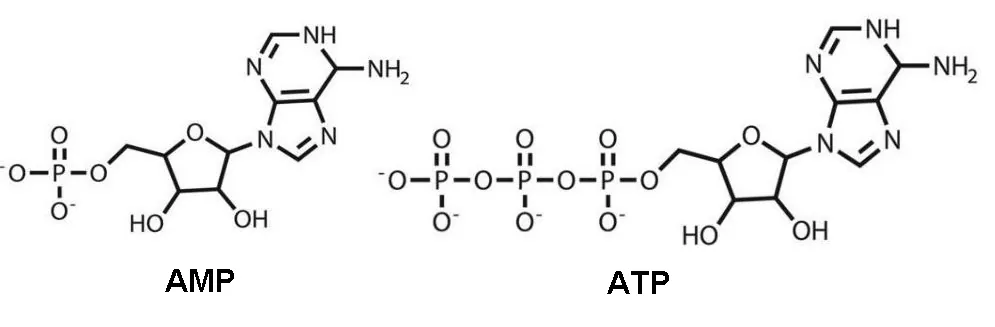
The conversion of ATP to ADP and AMP is what supplies cells with energy. Both of these molecules increase during fasting and exercise.
AMPK can bind to both AMP and ATP. If AMP binding happens then AMPK sends signals. These signals help the body use stored energy and slow down cell growth and division.
If AMPK binds to ATP, this doesn’t happen. The amounts of AMPs, ADPs, and ATPs change. This depends on whether the body has too little or too much energy.
AMPK is affected by many other molecules, including ALA, which has been shown to increase energy expenditure by enhancing AMPK-PGC-1α signaling [17]. PGC-1α is the master regulator of mitochondrial biogenesis [18], and ALA helps AMPK respond better to PGC-1α. This increases mitochondria in skeletal muscle, enabling the cell to burn up triglycerides for energy.
Enhancing mitochondrial biogenesis also likely lowers serum triglycerides and cholesterol because the cells’ ability to use triglycerides for energy is enhanced [17]. This also offers an explanation for why ALA would help promote weight loss, as fat is stored triglyceride [19].
Paradoxically, in the hypothalamus, ALA affects eating behavior by inhibiting AMPK, which decreases appetite [20, 21].
Alpha-lipoic acid and diabetes
Diabetes mellitus is a multifaceted metabolic disorder in which oxidative stress plays a major role. This has prompted several studies into the use of ALA as a therapeutic adjunct in the treatment of diabetes. In many of these studies, the clearest benefit of lipoic acid supplementation is in patients with diabetic neuropathy [22].
When insulin attaches to its receptor, a series of reactions happen. This leads to the movement of molecules that carry glucose into the cell. ALA has been found to activate the insulin signaling cascade in cultured cells [23].
Alpha-lipoic acid and cardiovascular disease
Mechanical and chemical factors, such as high blood pressure and smoking, can harm the cardiovascular system. They cause oxidative damage to blood vessels, air sacs, and heart muscle.
For instance, chronic hypertension elicits maladaptive changes in how the cells of the heart and blood vessels function [24]. These changes often initiate upwardly spiraling levels of oxidative damage and dysregulation of redox-based cellular communication systems.
Evidence suggests that ALA can both mitigate oxidative damage and help resolve abnormalities in redox-based cellular communication. The mechanisms include stopping free radicals, preventing T-cell infiltration, and restoring normal calcium and nitric oxide function. It also increases the activity of genes like ALDH2 that help to detoxify the system [13].
ALA directly quenches free radicals produced in the cardiovascular system. Additionally, ALA also maintains numerous radical scavenging enzymes; vitamins A, C, and E; and glutathione [25, 26]. For example, when a molecule of glutathione quenches a free radical reaction, glutathione becomes oxidized.
To re-use glutathione, a stronger antioxidant like ALA must change oxidized glutathione back to its original state. This allows it to keep fighting free radicals.
ALA dissolves in lipids, making it an antioxidant, and it can stop lipid peroxidation. This process happens when oxidants, like free radicals, attack lipids. These lipids have carbon-carbon double bonds, especially polyunsaturated fatty acids. Lipid peroxidation is especially important in heart, brain, and nervous system cells.
The infrequent replacement of these cells results in their membranes accumulating oxidized lipids [5], such as lipofuscin, which can be used as an aging biomarker [27]. In fact, ALA has been shown to reduce lipofuscin accumulation in rat brain tissue [28].
ALA reduces T-cell infiltration in atherosclerotic lesions. It is believed that this effect occurs by blocking molecules called vascular cell adhesion molecules (VCAM-1) and intercellular adhesion molecules (ICAM-1) [29, 30].
These molecules help parts of the immune system, like T cells, to attach and enter tissues. Stopping T cells from entering atherosclerotic lesions prevents more oxidative damage. It also stops the buildup of macrophage foam cells in the artery wall.
Foam cells can eventually cause arterial occlusion, resulting in stroke or heart attack [31]. Oxidative damage, in this case, is caused by immune cells, which use oxidation to destroy pathogens [32].
Pathogens are not always a main cause of atherosclerotic lesions. However, they trigger a general immune defense. This defense can cause a lot of damage, especially when the immune response is chronic.
ALA can aid in the mitigation of drug-induced calcium dysregulation. ALA offsets the side effects of drugs that activate β-adrenergic receptors, which, when activated, increase cardiovascular, pulmonary, metabolic, and central nervous system activity.
This has been shown to raise oxidative stress, heart inflammation, and clotting. It can also cause calcium overload in heart muscle, increasing the risk of heart attack [13].
ALA has been shown to restore endothelial nitric oxide bioavailability and function in the lining of blood vessels. Nitric oxide (NO) relaxes smooth muscles in the arterial wall to lower blood pressure.
Additionally, it prevents platelet activation and thereby clot formation. Finally, NO can lower blood pressure and reduce the heart’s workload. It does this by decreasing the force of contraction in heart muscle [33].
ALA and DHLA can affect the expression of important genes related to cardiovascular health. Aldehyde dehydrogenase 2 (ALDH2) and Cx3cll play key roles in the cellular regrowth that happens in the walls of blood vessels after an injury.
ALDH2 is an enzyme that detoxifies aldehyde compounds in the body [13]. Evidence suggests that ALA and DHLA increase ALDH2, enabling cells to detoxify aldehydes more effectively. Although there is some controversy, it is thought that ALDH2 may help reduce ischemia/reperfusion injury.
This is especially important in Asian communities, in which 40% of people may have mutations in the ALDH gene. These mutations can make them more vulnerable to aldehyde toxicity [34, 35].
ALA has been shown to reverse age-related changes in CCL2, thus helping to recruit immune cells. ALA also affects NF-κB, an important regulator of inflammation. It also influences Vcam, which allows immune cells to stick to blood vessel walls [30,36].
ALA and neurodegenerative disease
ALA helps protect against multiple sclerosis (MS), Parkinson’s disease, Alzheimer’s disease, stroke, and spinal cord injury [16].
ALA is thought to help with neurodegenerative diseases in several ways. It may prevent lipid peroxidation [5], can regulate T-cell blockage [30], and its effects on NF-κB inhibit matrix metalloproteinase 9 (MMP) [37, 38]. NF-κB is a central mediator in the inflammatory process [39], and MMP-9 plays a special role in nerve and brain tissue.
Lipid peroxidation is a major threat to brain and nervous system tissue for several reasons. First, neurons are long-lived cells whose membranes can suffer long-term damage from the buildup of oxidation in the lipid membrane. Second, the brain cells have a lot of polyunsaturated fatty acids, which are especially vulnerable to oxidation [40]. Third, the breakdown products of lipid peroxidation, often aldehydes, are also toxic to cells [5]. Lipid peroxidation has been implicated in the pathology of Parkinson’s disease and Alzheimer’s disease, among others [41].
MS is an autoimmune disease in which damage occurs from persistent immune system attacks on the brain and nerves. ALA is thought to change how cell adhesion molecules work. This includes molecules like ICAM-1 and VCAM-1 in nervous system tissue. This lowers T-cell infiltration, which decreases the entry of other immune system components that destroy nerve cells [29, 30].
The extracellular matrix (ECM), the material between cells, has a constantly changing composition. In the nervous system, MMP-9 participates in the remodeling of ECM. This remodeling process involves keeping myelin intact. Myelin is the outer layer of nerve cells and is important for nerve function and making neural connections [37].
Dysregulated activation of MMP-9 is implicated in multiple sclerosis [42]. It is also associated with traumatic brain injury and Alzheimer’s disease [37]. Therefore, ALA may short-circuit the process of neurodegeneration and demyelination.
With fewer immune cells present, reactive oxygen species (ROS) production declines. Immune cells actively make ROS as part of their pathogen response mechanism. In short, reducing inflammation reduces ROS in tissues.
ALA is an antioxidant that is adept at preventing lipid peroxidation, which occurs in cellular membranes. Preventing lipid peroxidation stops the buildup of its by-products, which include harmful aldehydes like acrolein [5, 43].
ALA treatment in rats following induced strokes was shown to be neuroprotective and facilitated recovery, reducing oxidative damage [44].
Alpha-lipoic acid and cancer
Oxidative stress is linked to tumor development. Antioxidants play a critical role in the mitigation of free radical-related diseases. ALA has been shown to help patients with advanced cancers by reducing ROS and increasing glutathione peroxidase activity. Unfortunately, dosage and bioavailability issues undermine the utility of most antioxidants that might otherwise be useful in cancer treatment.
In a tissue culture study, ALA was shown to inhibit the growth of breast cancer cells. The research team concluded that ALA inhibited breast cancer cell proliferation and induced cancer cells to self-destruct [45]. More recent studies have looked at delivering ALA to liposomes in order to improve cancer cell targeting and bioavailability. In most instances, the inclusion of ALA significantly enhanced the performance of traditional cancer drugs [14].
Alpha-lipoic acid and COVID-19
Oxidative stress is thought to play a role in viral infections. ALA’s antioxidant and immunomodulatory effects are well understood. As a result, ALA has been tested against many chronic immune diseases [46].
ALA also shows promise as a therapy applied after initial treatment for COVID-19 patients. In addition to lowering oxidative stress and protecting blood vessel linings, it can reduce the entry of SARS-CoV-2. It also decreases inflammation and can indirectly boost the immune system [47].
Alpha-lipoic acid and longevity
Animal studies show that alpha-lipoic acid prolonged lifespan in certain species [24]. However, it also reduced lifespan in progeric mice [25]. There is currently no data supporting any effect on human lifespan, and studies of its long-term effects on health have not been conducted.
Alpha-lipoic acid side effects
Taken as a supplement, ALA is generally considered safe, with no serious side effects reported. Occasionally, some people may experience mild symptoms like nausea, rashes, and itching. If you experience any serious adverse effects, cease taking ALA immediately and consult your doctor.
Disclaimer
This article is only a summary, it is not intended as an exhaustive guide. It is based on the interpretation of research data, which is speculative by nature. This article is not a substitute for consulting your physician about which supplements may or may not be right for you. We do not endorse supplement use nor any product or supplement vendor.
Literature
[1] E. E. Snell, F. M. Strong, and W. H. Peterson, “Growth Factors for Bacteria Fractionation and Properties of an Accessory Factor for Lactic Acid Bacteria’,” Biochem, vol. 31, pp. 1780–1799, 1937.
[2] R. L. J., D. B. G., G. I. C., and H. C. S., “Crystalline α-Lipoic Acid: A Catalytic Agent Associated with Pyruvate Dehydrogenase,” Science (80-. )., vol. 114, no. 2952, pp. 93–94, Jul. 1951.
[3] B. Salehi et al., “Insights on the Use of α-Lipoic Acid for Therapeutic Purposes,” Biomolecules, vol. 9, no. 8, p. 356, Aug. 2019
[4] V. Serhiyenko et. al., “Alpha-lipoic acid: mechanisms of action and beneficial effects in the prevention and treatment of diabetic complications,” MOJ Public Heal., vol. 7, no. 4, pp. 174–178, 2018
[5] A. Catalá and M. Díaz, Editorial: Impact of lipid peroxidation on the physiology and pathophysiology of cell membranes, vol. 7, no. SEP. 2016.
[6] P. Hasanein and A. Emamjomeh, “Chapter 28 – Beneficial Effects of Natural Compounds on Heavy Metal–Induced Hepatotoxicity,” R. R. Watson and V. R. B. T.-D. I. in L. D. Preedy, Eds. Academic Press, 2019, pp. 345–355.
[7] J. A. Mayr, R. G. Feichtinger, F. Tort, A. Ribes, and W. Sperl, “Lipoic acid biosynthesis defects,” J. Inherit. Metab. Dis., vol. 37, no. 4, pp. 553–563, Jul. 2014
[8] G. K. Kikuchi, Y. M. Otokawa,, “Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia,” vol. 84, pp. 246–263, 2008.
[9] M. Quick and L. Shi, “Chapter Three – The Sodium/Multivitamin Transporter: A Multipotent System with Therapeutic Implications,” in Hormones and Transport Systems, vol. 98, G. B. T.-V. & H. Litwack, Ed. Academic Press, 2015, pp. 63–100.
[10] E. Hanschmann et. al., “Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling,” Antioxidants Redox Signal., vol. 19, no. 13, pp. 1539–1605, 2013.
[11] M. C. Towler and D. G. Hardie, “AMP-activated protein kinase in metabolic control and insulin signaling,” Circ. Res., vol. 100, no. 3, pp. 328–341, 2007
[12] N. Najafi, S. Mehri, M. Ghasemzadeh Rahbardar, and H. Hosseinzadeh, “Effects of alpha lipoic acid on metabolic syndrome: A comprehensive review.,” Phytother. Res., 2022, doi: 10.1002/ptr.7406.
[13] Q.-F. Hu and A.-J. Sun, “Cardioprotective Effect of Alpha-lipoic Acid and its Mechanisms,” Cardiol. Plus, vol. 5, no. 3, 2020
[14] M. Attia, E. A. Essa, R. M. Zaki, and A. A. Elkordy, “An overview of the antioxidant effects of ascorbic acid and alpha lipoic acid (In liposomal forms) as adjuvant in cancer treatment,” Antioxidants, vol. 9, no. 5, pp. 1–15, 2020
[15] D. P. Tomer, L. D. McLeman, S. Ohmine, P. M. Scherer, B. K. Murray, and K. L. O’Neill, “Comparison of the total oxyradical scavenging capacity and oxygen radical absorbance capacity antioxidant assays,” J. Med. Food, vol. 10, no. 2, pp. 337–344, 2007
[16] H. Khan, T. G. Singh, R. S. Dahiya, and M. M. Abdel-Daim, “α-Lipoic Acid, an Organosulfur Biomolecule a Novel Therapeutic Agent for Neurodegenerative Disorders: An Mechanistic Perspective,” Neurochem. Res., 2022
[17] Y. Wang, X. Li, and Y. Guo, “Alpha-lipoic acid increases energy expenditure by enhancing AMPK-PGC-1alpha signaling in the skeletal muscle of aged mice,” Metabolism, vol. 59, no. 7, pp. 967–976, 2010
[18] H. Liang and W. F. Ward, “PGC-1α: A key regulator of energy metabolism,” Am. J. Physiol. – Adv. Physiol. Educ., vol. 30, no. 4, pp. 145–151, 2006
[19] E. H. Koh et al., “Effects of alpha-lipoic acid on body weight in obese subjects,” Am. J. Med., vol. 124, no. 1, pp. 85.e1-85.e8, 2011
[20] U. Andersson et al., “Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity,” Biochem. Biophys. Res. Commun., vol. 329, no. 2, pp. 719–725, 2005
[21] M.-S. Kim et al., “Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase,” Nat. Med., vol. 10, no. 7, pp. 727–733, 2004
[22] S. Golbidi, M. Badran, and I. Laher, “Diabetes and alpha lipoic Acid,” Front. Pharmacol., vol. 2, p. 69, Nov. 2011
[23] D. Konrad, “Utilization of the Insulin-Signaling Network in the Metabolic Actions of α-Lipoic Acid—Reduction or Oxidation?,” Antioxid. Redox Signal., vol. 7, no. 7–8, pp. 1032–1039, Jul. 2005
[24] S. Laurent, “https://pubmed.ncbi.nlm.nih.gov/7635546/,” Hypertension, vol. 26, no. 2, pp. 355–362, Aug. 1995
[25] W. Jones, X. Li, Z. Qu, L. Perriott, R. R. Whitesell, and J. M. May, “Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells,” Free Radic. Biol. Med., vol. 33, no. 1, pp. 83–93, 2002
[26] J. Zhang. et al., “Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid,” Proc. Natl. Acad. Sci., vol. 101, no. 10, pp. 3381–3386, Mar. 2004
[27] E. Kaemmerer et. al., “Effects of Lipid Peroxidation-Related Protein Modifications on RPE Lysosomal Functions and POS Phagocytosis,” Invest. Ophthalmol. Vis. Sci., vol. 48, no. 3, pp. 1342–1347, Mar. 2007
[28] P. Arivazhagan and C. Panneerselvam, “Alpha-Lipoic Acid Increases Na+K+ATPase Activity and Reduces Lipofuscin Accumulation in Discrete Brain Regions of Aged Rats,” Ann. N. Y. Acad. Sci., vol. 1019, no. 1, pp. 350–354, Jun. 2004
[29] M. Sans et al., “VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis,” Gastroenterology, vol. 116, no. 4, pp. 874–883, 1999
[30] W. Liu, L. J. Shi, and S. G. Li, “The Immunomodulatory Effect of Alpha-Lipoic Acid in Autoimmune Diseases,” Biomed Res. Int., vol. 2019, 2019
[31] X. H. Yu, Y. C. Fu, D. W. Zhang, K. Yin, and C. K. Tang, “Foam cells in atherosclerosis,” Clin. Chim. Acta, vol. 424, pp. 245–252, 2013
[32] A. Dumas and U. G. Knaus, “Raising the ‘Good’ Oxidants for Immune Protection ,” Frontiers in Immunology , vol. 12. 2021.
[33] T. Heitzer, B. Finckh, S. Albers, K. Krohn, A. Kohlschütter, and T. Meinertz, “Beneficial effects of α-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress,” Free Radic. Biol. Med., vol. 31, no. 1, pp. 53–61, 2001
[34] J. Zhang et. al.,, “Alpha-Lipoic Acid Ameliorates Oxidative Stress by Increasing Aldehyde Dehydrogenase-2 Activity in Patients with Acute Coronary Syndrome,” Tohoku J. Exp. Med., vol. 229, no. 1, pp. 45–51, 2013
[35] V. Vasiliou and D. Petersen, “4.07 – Aldehyde Dehydrogenases,” (Second E. McQueen, Ed. Oxford: Elsevier, 2010, pp. 131–147.
[36] F. Seifar et al., “α-Lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: A review,” Nutr. Neurosci., vol. 22, no. 5, pp. 306–316, 2019
[37] S. Reinhard, K. Razak, and I.Ethell, “A delicate balance: role of MMP-9 in Polyunsaturated Fatty Acid Metabolism in the Brain and Brain Cells,” Frontiers in Cellular Neuroscience, vol. 9. 2015.
[38] H. S. Kim et al., “α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity,” Exp. Mol. Med., vol. 39, no. 1, pp. 106–113, 2007, doi: 10.1038/emm.2007.12.
[39] T. Liu, L. Zhang, and S. Sun, “NF-κB signaling in inflammation,” Signal Transduct. Target. Ther., vol. 2, p. 17023, 2017
[40] C. J. E.-C. B.-B. E.-S. L. E.-V. Pallet, “Polyunsaturated Fatty Acid Metabolism in the Brain and Brain Cells,” Rijeka: IntechOpen, 2019, p. Ch. 2.
[41] D. De Araújo et al., “Behavioral and neurochemical effects of alpha-lipoic acid in the model of parkinson’s disease induced by unilateral stereotaxic injection of 6-ohda in rat,” Evidence-based Complement. Altern. Med., vol. 2013, 2013
[42] A. Trentini et al., “Interplay between Matrix Metalloproteinase-9, Matrix Metalloproteinase-2, and Interleukins in Multiple Sclerosis Patients,” Dis. Markers, vol. 2016, p. 3672353, 2016
[43] K. Nickander, B.Mcphee, P. Low, and H. Tritschler, “Alpha-lipoic acid: Antioxidant potency against lipid peroxidation of neural tissues in vitro and implications for diabetic neuropathy,” Free Radic. Biol. Med., vol. 21, no. 5, pp. 631–639, 1996
[44] C. Lv et al., “Alpha-Lipoic Acid Promotes Neurological Recovery After Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage.” pp. 1273–1286, 2017.
[45] M.Na, E. Seo, and W. Kim, “Effects of α-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells,” Nutr. Res. Pract., vol. 3, no. 4, p. 265, 2009
[46] S. Dragomanova et al., “Therapeutic Potential of Alpha-Lipoic Acid in Viral Infections, including COVID-19,” Antioxidants , vol. 10, no. 8. 2021.
[47] L. Rochette and S. Ghibu, “Mechanics Insights of Alpha-Lipoic Acid against Cardiovascular Diseases during COVID-19 Infection,” International Journal of Molecular Sciences , vol. 22, no. 15. 2021


