Mitochondrial Dysfunction
Mitochondrial dysfunction is one of the major components of aging as described in the Hallmarks of Aging [1]. As they age, mitochondria lose their ability to provide cellular energy, and they release reactive oxygen species that harm cells.
What are mitochondria?
Mitochondria, which are often called the powerhouses of cells, act like miniature factories, converting the food we eat into usable energy in the form of a chemical called adenosine triphosphate (ATP) [2]. ATP provides energy to fuel a myriad of cellular processes, such as muscle contraction, nerve impulse propagation, and protein synthesis. ATP is common to all forms of life and is often referred to as the “molecular unit of currency” of intracellular energy transfer.
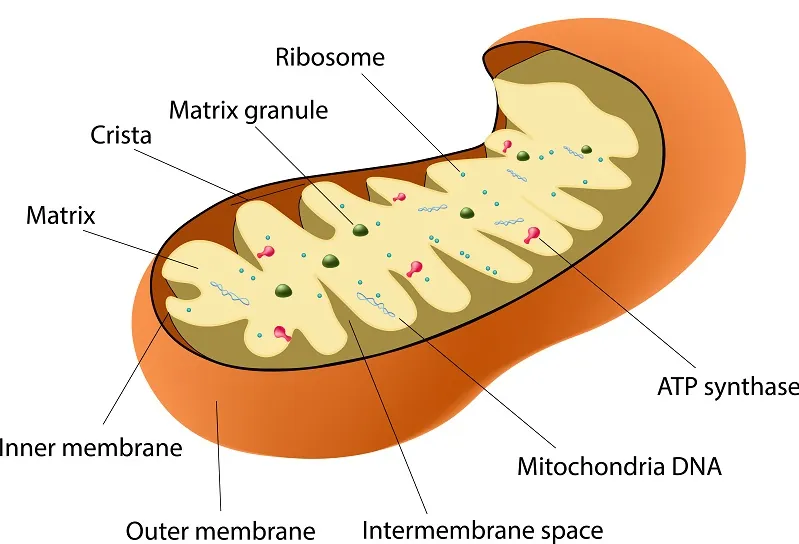
Interestingly, mitochondria did not originate as part of multicellular life; they are stowaways in our cells and have their own unique DNA, which is separate from our own. It is widely thought that they merged with a very early ancestor of all multicellular life to form a symbiotic relationship [3]. Mitochondria become dysfunctional as we age and are host to their own separate (though similar) forms of damage.
How do mitochondria become dysfunctional?
As we age, our mitochondria go through changes that harm their ability to provide us with chemical energy while causing the release of harmful reactive oxygen species (ROS) [4], which can cause DNA mutations leading to cancer [5, 6] and even harm proteostasis [7]. ROS also drive muscle weakness [8], a further smoldering level of background inflammation (inflammaging) [9], along with the associated bone frailty [10], senescent cell load [11] and immune suppression [12] of old age. Mitochondria from elderly people even look different [13]: they swell while their numbers dwindle, unable to replace themselves as quickly in their dysfunctional state [14, 15].
These problems aren’t all that ROS can cause, however; they can also cause mutations in mitochondrial DNA [16]. While some studies suggest that this damage is not done directly [17], ROS can damage the very proteins that would control the reproduction of mitochondria and introduce additional errors into the copies by extension [7].
While most of these issues are detected by quality-control mechanisms in the cell [18], causing damaged mitochondria to be destroyed through a process called mitophagy, these systems become less and less effective with age, decreasing in activity and eventually allowing errors to slip through. In some cases, this isn’t so bad; each cell contains many mitochondria, so ten or even a few hundred being mutated isn’t a problem. However, some of these errors can make the dysfunctional mitochondria survive longer than healthy mitochondria. In this way, some types of dysfunctional mitochondria build up and eventually become more common than healthy ones [19].
According to biogerontologist Aubrey de Grey, this phenomenon is known as the “Survival of the Slowest” hypothesis. This theory suggests that some mitochondria become so impaired that they produce less ROS than healthier, more active mitochondria.
Since high ROS levels are one of the signals that prompt mitophagy, these “slower” or more dysfunctional mitochondria can evade the cell’s quality-control system, accumulating within cells rather than being cleared away. Over time, these sluggish mitochondria build up, taking up space and resources while contributing less energy to the cell. As they accumulate, they perpetuate a vicious cycle, adding to cellular dysfunction and general aging.
This accumulation of dysfunctional mitochondria ultimately impairs cellular function, contributing to various signs of aging and age-related diseases. By understanding this “Survival of the Slowest” mechanism, scientists hope to develop therapies to enhance it [20].
Additionally, as aging progresses, NAD+ levels in human cells decrease, causing a breakdown in communication between the human nucleus and mitochondrial DNA, again leading to decreased energy production and increased ROS production [21].
Mitochondrial dysfunction across systems and diseases
Recent studies have expanded our understanding of mitochondrial dysfunction, illustrating its role in different pathological contexts. A growing body of evidence emphasizes the organ- and system-specific consequences of impaired mitochondrial function, offering new insights into its mechanisms and therapeutic potential.
Mitochondrial dysfunction has been increasingly recognized as a critical factor in vascular endothelial injury, where it disrupts endothelial cell homeostasis through dysregulated mitochondrial dynamics and oxidative stress. These changes contribute to the pathogenesis of atherosclerosis, hypertension, and diabetes by promoting endothelial apoptosis and impairing cellular energy metabolism [22]. Concurrently, research has highlighted the centrality of mitochondrial dysfunction in brain aging, where it drives chronic inflammation, oxidative stress, and excitotoxicity. These factors collectively degrade neural networks, Disruptions in mitochondrial function lead to a decline in the efficiency of neural communication, where beneficial signals in the brain become less distinct amid background activity. This diminished clarity in neural signaling contributes to the development and progression of neurodegenerative diseases [23].
The liver is another organ profoundly affected by mitochondrial impairment. Mitochondrial dysfunction plays a pivotal role in non-alcoholic fatty liver disease, viral hepatitis, and hepatocellular carcinoma. This dysfunction manifests as excessive ROS production, mitochondrial DNA damage, and impaired bioenergetics, leading to hepatocyte inflammation and programmed cell death [24]. In enterocytes, mitochondrial dysfunction disrupts dietary lipid processing by impairing chylomicron production and transport. This effect results in lipid accumulation and highlights the critical role of mitochondria in gastrointestinal health [25].
Age-related disorders also exhibit a strong link to mitochondrial dysfunction, which accelerates cellular aging through the accumulation of ROS, a decline in mitophagy, and disruption of mitochondrial membrane integrity. Therapeutic strategies targeting mitochondrial repair and rejuvenation, such as antioxidants and mitophagy stimulators, are being explored to mitigate these effects [26]. Similarly, in the context of sepsis, mitochondrial damage has been implicated in the dysregulation of immune responses and multi-organ failure. Damaged mitochondria amplify inflammation through signaling pathways that promote septic pathogenesis, underscoring the therapeutic promise of mitochondrial-targeted interventions in critical care settings [27].
Emerging treatments for neurodegenerative diseases, including Alzheimer’s and Parkinson’s diseases, are leveraging mitochondria-targeted strategies. These include enhancing mitochondrial fusion, biogenesis, and antioxidant defenses to counteract oxidative stress and maintain mitochondrial homeostasis [28]. Organ-specific approaches to mitochondrial dysfunction have also gained traction. Recent work highlights the necessity of tailoring therapeutic interventions to the unique mitochondrial biology of different tissues, such as the brain, liver, and skeletal muscle. These efforts aim to develop precision medicine strategies that restore mitochondrial function and mitigate aging [29].
Together, these findings underscore the pervasive role of mitochondrial dysfunction in aging and disease. A deeper understanding of the molecular mechanisms underlying mitochondrial impairment and its systemic effects will be critical in developing effective interventions to promote health and longevity.
How could we prevent or reverse this?
In addition to NAD⁺ supplementation, targeting mitochondrial oxidative stress with mitochondria-targeted antioxidants has shown promise. Compounds such as MitoQ and SkQ1 are designed to accumulate within mitochondria and neutralize ROS at the source. Clinical trials have indicated that reducing oxidative stress and improving mitochondrial function are potentially effective against age-related diseases [30, 31].
Enhancing mitophagy is another strategy. Urolithin A, derived from dietary polyphenols, has been shown to induce mitophagy [32, 33].
Gene therapy approaches, such as CRISPR/Cas9 and mitochondrial-targeted nucleases like mitoTALENs, are being explored to correct mutations in mitochondrial DNA (mtDNA) that accumulate with age and impair mitochondrial function. Scientists have demonstrated the potential of these technologies in treating mitochondrial diseases and mitigating age-related decline [34, 35].
Moreover, moving the most vital parts of the mitochondrial DNA to the nucleus could provide better DNA repair mechanisms and keep them away from the source of ROS. This approach has been demonstrated to work with some of this vital code [36]. Of note, the study proving that this is possible was crowdfunded on Lifespan.io!
Lifestyle interventions, such as regular exercise and dietary modifications, also play a significant role in enhancing mitochondrial health. Regular physical activity is one of the most effective natural stimulators of mitochondrial biogenesis and function. High-intensity interval training (HIIT) and resistance training are particularly beneficial in enhancing mitochondrial health in older adults [37, 38].
Finally, ongoing research into pharmacological agents that target mitochondrial biogenesis, such as PGC-1α activators, offers additional avenues for intervention. By promoting the formation of new mitochondria, these agents can enhance cellular energy capacity and potentially mitigate age-related mitochondrial dysfunction [39, 40].
In conclusion, addressing mitochondrial dysfunction involves a multifaceted approach, combining pharmacological, genetic, and lifestyle strategies. Continued research and innovation in this field hold the promise of enhancing mitochondrial health, thereby improving overall longevity and quality of life.
Conclusion
Mitochondrial dysfunction is an important part of aging. These miniature chemical engines, while capable of self-replication, gradually become more dysfunctional with age through a variety of mechanisms, causing harm to our cells and encouraging more dysfunction in a vicious cycle. Quality control mechanisms hold this at bay for a time, but they eventually fail, leading to multiple diseases of aging and a long-lasting, chronic background level of inflammation called inflammaging.
Literature
[1] López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194.
[2] Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol 2020, 37, 101674.
[3] Gray, M.W.; Burger, G.; Lang, B.F. The Origin and Early Evolution of Mitochondria. Genome Biology 2001 2:6 2001, 2, 1–5.
[4] Lesnefsky, E.J.; Hoppel, C.L. Oxidative Phosphorylation and Aging. Ageing Res Rev 2006, 5, 402–433.
[5] Basu, A.K. DNA Damage, Mutagenesis and Cancer. Int J Mol Sci 2018, 19, 970.
[6] McAdam, E.; Brem, R.; Karran, P. Oxidative Stress-Induced Protein Damage Inhibits DNA Repair and Determines Mutation Risk and Therapeutic Efficacy. Mol Cancer Res 2016, 14, 612–622.
[7] Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, Oxidative Stress and Aging. Redox Biol 2017, 13, 550–567.
[8] Alway, S.E.; Mohamed, J.S.; Myers, M.J. Mitochondria Initiate and Regulate Sarcopenia. Exerc Sport Sci Rev 2017, 45, 58–69.
[9] Rimessi, A.; Previati, M.; Nigro, F.; Wieckowski, M.R.; Pinton, P. Mitochondrial Reactive Oxygen Species and Inflammation: Molecular Mechanisms, Diseases and Promising Therapies. Int J Biochem Cell Biol 2016, 81, 281–293.
[10] Lane, R.K.; Hilsabeck, T.; Rea, S.L. The Role of Mitochondrial Dysfunction in Age-Related Diseases. Biochim Biophys Acta 2015, 1847, 1387–1400.
[11] Kamogashira, T.; Hayashi, K.; Fujimoto, C.; Iwasaki, S.; Yamasoba, T. Functionally and Morphologically Damaged Mitochondria Observed in Auditory Cells under Senescence-Inducing Stress. npj Aging and Mechanisms of Disease 2017 3:1 2017, 3, 1–11.
[12] Frasca, D.; Blomberg, B.B. Inflammaging Decreases Adaptive and Innate Immune Responses in Mice and Humans. Biogerontology 2016, 17, 7–19.
[13] Gerencser, A.A.; Doczi, J.; Töröcsik, B.; Bossy-Wetzel, E.; Adam-Vizi, V. Mitochondrial Swelling Measurement in Situ by Optimized Spatial Filtering: Astrocyte-Neuron Differences. Biophys J 2008, 95, 2583–2598.
[14] Seo, A.Y.; Joseph, A.M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New Insights into the Role of Mitochondria in Aging: Mitochondrial Dynamics and More. J Cell Sci 2010, 123, 2533–2542.
[15] Figge, M.T.; Reichert, A.S.; Meyer-Hermann, M.; Osiewacz, H.D. Deceleration of Fusion–Fission Cycles Improves Mitochondrial Quality Control during Aging. PLoS Comput Biol 2012, 8, e1002576.
[16] Lee, H.-C.; Wei, Y.-H. Oxidative Stress, Mitochondrial DNA Mutation, and Apoptosis in Aging. https://doi.org/10.3181/00379727-232-2320592 2007.
[17] Itsara, L.S.; Kennedy, S.R.; Fox, E.J.; Yu, S.; Hewitt, J.J.; Sanchez-Contreras, M.; Cardozo-Pelaez, F.; Pallanck, L.J. Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations. PLoS Genet 2014, 10.
[18] Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes (Basel) 2017, 8.
[19] Luo, C.; Li, Y.; Wang, H.; Feng, Z.; Li, Y.; Long, J.; Liu, J. Mitochondrial Accumulation under Oxidative Stress Is Due to Defects in Autophagy. J Cell Biochem 2013, 114, 212–219.
[20] De Grey, A.D.N.J. A Proposed Refinement of the Mitochondrial Free Radical Theory of Aging. BioEssays 1997, 19, 161–166.
[21] Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155, 1624.
[22] Pang, B.; Dong, G.; Pang, T.; Sun, X.; Liu, X.; Nie, Y.; Chang, X. Emerging Insights into the Pathogenesis and Therapeutic Strategies for Vascular Endothelial Injury-Associated Diseases: Focus on Mitochondrial Dysfunction. Angiogenesis 2024, 27.
[23] Bondy, S.C. Mitochondrial Dysfunction as the Major Basis of Brain Aging. Biomolecules 2024, 14.
[24] Chen, P.; Yao, L.; Yuan, M.; Wang, Z.; Zhang, Q.; Jiang, Y.; Li, L. Mitochondrial Dysfunction: A Promising Therapeutic Target for Liver Diseases. Genes Dis 2023, 11, 101115.
[25] Moschandrea, C.; Kondylis, V.; Evangelakos, I.; Herholz, M.; Schneider, F.; Schmidt, C.; Yang, M.; Ehret, S.; Heine, M.; Jaeckstein, M.Y.; et al. Mitochondrial Dysfunction Abrogates Dietary Lipid Processing in Enterocytes. Nature 2023, 625, 385.
[26] Somasundaram, I.; Jain, S.M.; Blot-Chabaud, M.; Pathak, S.; Banerjee, A.; Rawat, S.; Sharma, N.R.; Duttaroy, A.K. Mitochondrial Dysfunction and Its Association with Age-Related Disorders. Front Physiol 2024, 15, 1384966.
[27] Hu, D.; Sheeja Prabhakaran, H.; Zhang, Y.Y.; Luo, G.; He, W.; Liou, Y.C. Mitochondrial Dysfunction in Sepsis: Mechanisms and Therapeutic Perspectives. Critical Care 2024 28:1 2024, 28, 1–19.
[28] Choi, E.H.; Kim, M.H.; Park, S.J. Targeting Mitochondrial Dysfunction and Reactive Oxygen Species for Neurodegenerative Disease Treatment. International Journal of Molecular Sciences 2024, Vol. 25, Page 7952 2024, 25, 7952.
[29] Madreiter-Sokolowski, C.T.; Hiden, U.; Krstic, J.; Panzitt, K.; Wagner, M.; Enzinger, C.; Khalil, M.; Abdellatif, M.; Malle, E.; Madl, T.; et al. Targeting Organ-Specific Mitochondrial Dysfunction to Improve Biological Aging. Pharmacol Ther 2024, 262.
[30] Pham, T.; MacRae, C.L.; Broome, S.C.; D’souza, R.F.; Narang, R.; Wang, H.W.; Mori, T.A.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. MitoQ and CoQ10 Supplementation Mildly Suppresses Skeletal Muscle Mitochondrial Hydrogen Peroxide Levels without Impacting Mitochondrial Function in Middle-Aged Men. European Journal of Applied Physiology 2020 120:7 2020, 120, 1657–1669.
[31] Huang, B.; Zhang, N.; Qiu, X.; Zeng, R.; Wang, S.; Hua, M.; Li, Q.; Nan, K.; Lin, S. Mitochondria-Targeted SkQ1 Nanoparticles for Dry Eye Disease: Inhibiting NLRP3 Inflammasome Activation by Preventing Mitochondrial DNA Oxidation. Journal of Controlled Release 2024, 365, 1–15.
[32] Faitg, J.; D’Amico, D.; Rinsch, C.; Singh, A. Mitophagy Activation by Urolithin A to Target Muscle Aging. Calcif Tissue Int 2024, 114, 53–59.
[33] Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A Improves Muscle Strength, Exercise Performance, and Biomarkers of Mitochondrial Health in a Randomized Trial in Middle-Aged Adults. Cell Rep Med 2022, 3.
[34] Moraes, C.T. Tools for Editing the Mammalian Mitochondrial Genome. Hum Mol Genet 2024, 33, R92–R99.
[35] Nikitchina, N.; Ulashchik, E.; Shmanai, V.; Heckel, A.M.; Tarassov, I.; Mazunin, I.; Entelis, N. Targeting of CRISPR-Cas12a CrRNAs into Human Mitochondria. Biochimie 2024, 217, 74–85.
[36] Boominathan, A.; Vanhoozer, S.; Basisty, N.; Powers, K.; Crampton, A.L.; Wang, X.; Friedricks, N.; Schilling, B.; Brand, M.D.; O’Connor, M.S. Stable Nuclear Expression of ATP8 and ATP6 Genes Rescues a MtDNA Complex V Null Mutant. Nucleic Acids Res 2016, 44, 9342–9357.
[37] Ruegsegger, G.N.; Pataky, M.W.; Simha, S.; Robinson, M.M.; Klaus, K.A.; Nair, K.S. High-Intensity Aerobic, but Not Resistance or Combined, Exercise Training Improves Both Cardiometabolic Health and Skeletal Muscle Mitochondrial Dynamics. J Appl Physiol 2023, 135, 763–774.
[38] Chrøis, K.M.; Dohlmann, T.L.; Søgaard, D.; Hansen, C.V.; Dela, F.; Helge, J.W.; Larsen, S. Mitochondrial Adaptations to High Intensity Interval Training in Older Females and Males. Eur J Sport Sci 2020, 20, 135–145.
[39] Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, Vol. 12, Page 1075 2023, 12, 1075.
[40] Villena, J.A. New Insights into PGC-1 Coactivators: Redefining Their Role in the Regulation of Mitochondrial Function and Beyond. FEBS Journal 2015, 282, 647–672.