Skin Collagen Loss Makes It Easier For Cancer To Grow
- Sparseness of collagen was directly correlated with tumor formation.
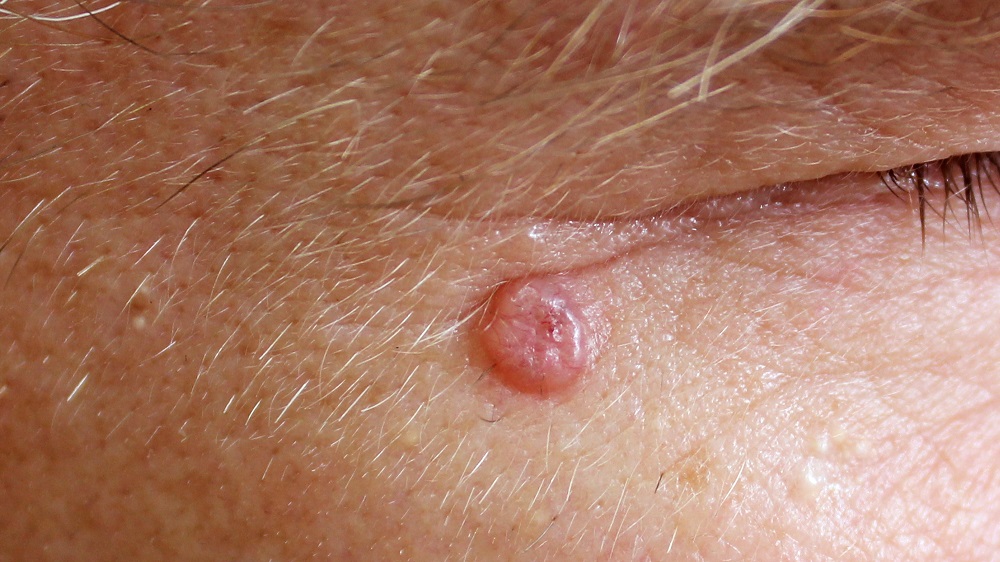
A new paper published in Nature has described how the aging extracellular matrix makes it easier for cancer to grow.
When cancer is skin deep
Under normal circumstances, we lose and regain skin cells constantly. Old skin flakes away, and stem cells constantly divide and differentiate to replace it. This is a homeostatic balance, and a disruption of this balance has been previously reported to lead to cancer, among other problems [1].
Basal cell carcinoma (BCC), the most common form of cancer in people, is caused by known mutations. Specifically, a loss of function of the Patched1 gene or a gain of function of the Smoothed gene leads to an activation of the Hedgehog pathway, leading to cancer [2]. Researchers have replicated this in mice through the mutant cancer-causing gene (oncogene) SmoM2 [3].
However, the skin is dividing constantly, and even oncogenes aren’t always enough to cause tumors to form. The cancer cells don’t always outcompete their normal counterparts, so some skin can look perfectly normal despite some of the cells being cancerous [4]. Therefore, these researchers looked for reasons why oncogenic cells outcompete normal cells, focusing on the cells’ environment.
One place but not another
In mice, SmoM2 cells form tumors in the tails and ears of mice, but not their backs [3]. To investigate this more closely, the researchers developed a mouse model in which Smo2 expression, along with a color change under fluorescent light, is instigated by tamoxifen. This let them visually observe the growth, or lack thereof, of this cancer under different conditions.
Just like in previous research [5], the SmoM2 cells in the ear were able to self-renew and divide rapidly, first expanding to the sides and then invading other skin layers. In the back, the vertical expansion was considerably diminished, but the horizontal expansion was greater. The stress of the cancer’s growth affected the shape of the normal cells in the ear but not the back. The SmoM2 cells in the back also induced terminal differentiation in the normal cells, a sign that they were being outcompeted.
The cancerous cells in the two areas also showed different levels of gene expression. In the ear, the researchers observed a substantial amount of gene upregulation that dovetails with BCC formation, including an increase in the Hedgehog protein and changes in extracellular matrix-related proteins, but these changes in gene expression did not exist in the back. Similarly, increased inflammation was found only in the ear after SmoM2 cells were introduced. The cancer cells were growing and proliferating, but they were not behaving like cancer.
A question of collagen
The researchers noted only a few proteins that were more abundant in the back than the skin tissue. Two of them were related to collagen formation. Collagen is considerably thicker in the backs than the ears of mice, and their skin is substantially thicker. The researchers surmise that this stiffness is the reason why the cancer cells are unable to form tumors.
These results were bolstered by more in-depth studies. The ear tissue of mice varies in thickness, and SmoM2 was less able to invade the more collagen-dense regions. Similarly, and critically, mice older than 18 months begin to lose collagen thickness in the same way as elderly humans. As expected, SmoM2 cells readily formed BCC in the backs of aged mice. Exposure to ultraviolet radiation, which depletes collagen, was found to have similar effects.
While this was not a human study, and human carcinomas may not always behave the same way as in mice, the researchers note that BCC increases with age in people as well. Further research will need to be done to determine how much collagen thickness affects the growth of this cancer and if collagen-improving treatments can help to prevent it.
Literature
[1] Blanpain, C., & Simons, B. D. (2013). Unravelling stem cell dynamics by lineage tracing. Nature reviews Molecular cell biology, 14(8), 489-502.
[2] Epstein, E. H. (2008). Basal cell carcinomas: attack of the hedgehog. Nature Reviews Cancer, 8(10), 743-754.
[3] Youssef, K. K., Van Keymeulen, A., Lapouge, G., Beck, B., Michaux, C., Achouri, Y., … & Blanpain, C. (2010). Identification of the cell lineage at the origin of basal cell carcinoma. Nature cell biology, 12(3), 299-305.
[4] Kakiuchi, N., & Ogawa, S. (2021). Clonal expansion in non-cancer tissues. Nature Reviews Cancer, 21(4), 239-256.
[5] Sánchez-Danés, A., Hannezo, E., Larsimont, J. C., Liagre, M., Youssef, K. K., Simons, B. D., & Blanpain, C. (2016). Defining the clonal dynamics leading to mouse skin tumour initiation. Nature, 536(7616), 298-303.








