A Ketosis-Related Compound Alleviates Arthritis in Rats
- This compound can be directly injected.
In Aging Cell, researchers have published details on the pathway by which a compound commonly found in ketone bodies ameliorates osteoarthritis in a rat model.
Chondrocytes are crucial in arthritis
The cartilage holding joints together is built and maintained by chondrocytes, which synthesize such necessary proteins as collagen and aggrecan [1]. Unfortunately, the cellular senescence that accompanies aging prevents these cells from proliferating and doing their jobs properly [2].
Metabolism has a substantial effect on chondrocyte function [3]. A review of human clinical trials has found that the ketone bodies that occur with the consumption of a ketogenic diet can alleviate osteoarthritis [4]; however, that previous work could not elaborate on the biochemical reasons why.
These researchers have noted that β-hydroxybutyrate (βOHB), a major component of ketone bodies [5], is substantially diminished in the knee joint fluid of osteoarthritis patients [6]. As βOHB has been found to suppress inflammation [7, 8] and affects fundamental AMPK metabolism [9], the researchers suspected that βOHB might play a causal role in suppressing osteoarthritis.
Less inflammation, better function
For the first experiment, the researchers subjected rats to a surgical procedure that induces arthritis or to a sham surgery, and then subdivided those two groups into standard-diet and ketosis-inducing diet groups. βOHB was very significantly elevated in rats fed a keto diet, whether they had induced arthritis or not. Of the rats fed a standard diet, the induced arthritis group had slightly less βOHB than the sham group.
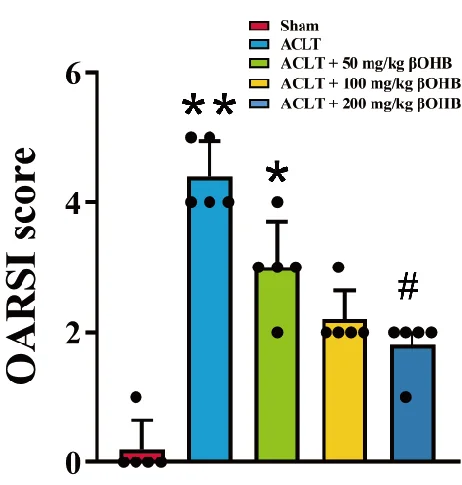
The keto diet reduced inflammatory factors in the arthritis-induced group. Compared to arthritic rats fed a standard diet, the ketosis group had less TNF-α, IL-6, and PGE2 while scoring better on the OARSI, a test that assesses function in osteoarthritis.
In another experiment, groups of rats were directly injected with βOHB in various concentrations instead of being fed a keto diet. The effects were substantial and dose-dependent. Once more, TNF-α, IL-6, and PGE2 were all substantially reduced, and OARSI scores were improved.
Cellular effects
The hydrogen peroxide derivative TBHP induces senescence and oxidative stress in chondrocytes and is commonly used to model arthritis on a cellular level. As such, it is highly toxic to these cells, regularly causing death by apoptosis. However, βOHB was able to protect chondrocytes from moderate doses of TBHP, neutralizing these toxic effects. The TBHP-induced senescence was substantially reduced, the secretions of senescent cells were similarly reduced, and the affected chrondrocytes were once able to produce cartilage-forming proteins. Apoptosis, too, was reduced, and an investigation found that the key proteins involved in apoptosis were strongly upregulated with TBHP but reduced with βOHB.
Previous work had found that mitochondrial maintenance is key in chondrocyte function and senescence prevention [10]. A main indicator of mitochondrial function that had nearly vanished in cells given TBHP had it restored by βOHB. Similarly, mitochondria given TBHP were often found in fragments, but administering βOHB prevented this fragmentation. While it had no significant effects on cells that were not subjected to TBHP, βOHB improved multiple metrics in the mitochondria of the cells that were, including ATP production and respiration.
This was found to be due to an increase in mitophagy, the process of cells consuming their own damaged mitochondria. Cells that were additionally given other compounds that interfered with the PINK1 mitophagy pathway could no longer be aided by βOHB. The researchers also found that this treatment requires HCAR2, a receptor of βOHB, to function properly, and that the AMPK pathway is crucial to its function; interference in either of these places also prevented βOHB from having its effects.
These results, while promising and positive, came from experiments done on a rat model and on cells, and they may or may not apply to patients in the clinic. Trials would have to be conducted to determine if direct administration of βOHB has any positive effects on people suffering from arthritis.
Literature
[1] Zhang, Y., Jin, W., Chen, J., Wei, S., Cai, W., Zhong, Y., … & Peng, H. (2023). Gastrodin alleviates rat chondrocyte senescence and mitochondrial dysfunction through Sirt3. International Immunopharmacology, 118, 110022.
[2] Chen, H., Wu, J., Wang, Z., Wu, Y., Wu, T., Wu, Y., … & Shang, S. (2021). Trends and patterns of knee osteoarthritis in China: a longitudinal study of 17.7 million adults from 2008 to 2017. International Journal of Environmental Research and Public Health, 18(16), 8864.
[3] Zheng, L., Zhang, Z., Sheng, P., & Mobasheri, A. (2021). The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing research reviews, 66, 101249.
[4] Abboud, M., AlAnouti, F., Georgaki, E., & Papandreou, D. (2021). Effect of ketogenic diet on quality of life in adults with chronic disease: A systematic review of randomized controlled trials. Nutrients, 13(12), 4463.
[5] Sharma, R., & Ramanathan, A. (2020). The aging metabolome—biomarkers to hub metabolites. Proteomics, 20(5-6), 1800407.
[6] Mickiewicz, B., Kelly, J. J., Ludwig, T. E., Weljie, A. M., Wiley, J. P., Schmidt, T. A., & Vogel, H. J. (2015). Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. Journal of Orthopaedic Research®, 33(11), 1631-1638.
[7] Fu, S. P., Li, S. N., Wang, J. F., Li, Y., Xie, S. S., Xue, W. J., … & Liu, J. X. (2014). BHBA suppresses LPS‐induced inflammation in BV‐2 cells by inhibiting NF‐κB activation. Mediators of inflammation, 2014(1), 983401.
[8] Youm, Y. H., Nguyen, K. Y., Grant, R. W., Goldberg, E. L., Bodogai, M., Kim, D., … & Dixit, V. D. (2015). The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nature medicine, 21(3), 263-269.
[9] Carretta, M. D., Barría, Y., Borquez, K., Urra, B., Rivera, A., Alarcón, P., … & Burgos, R. A. (2020). β-hydroxybutyrate and hydroxycarboxylic acid receptor 2 agonists activate the AKT, ERK and AMPK pathways, which are involved in bovine neutrophil chemotaxis. Scientific Reports, 10(1), 12491.
[10] Shang, J., Lin, N., Peng, R., Jiang, N., Wu, B., Xing, B., … & Lu, H. (2023). Inhibition of Klf10 attenuates oxidative stress-induced senescence of chondrocytes via modulating mitophagy. Molecules, 28(3), 924.







