Menopause: Causes and Health Consequences
Menopause is associated with the cessation of the female reproductive span. While this is true, it has far more consequences for the female body. The hormonal changes accompanying menopause are associated with declines in cognition, cardiovascular health, and other aspects of life.
Definitions
The World Health Organization (WHO) defines menopause “as the permanent cessation of menstrual periods due to the loss of ovarian activity without any other pathological or physiological cause“ [1].
Menopause can be defined retrospectively as a point in time when a woman does not have menstrual cycles for 12 months after her final menstrual period [2]. This usually occurs between 45 and 55 years of age, but it differs depending on many factors, including by population [3]:
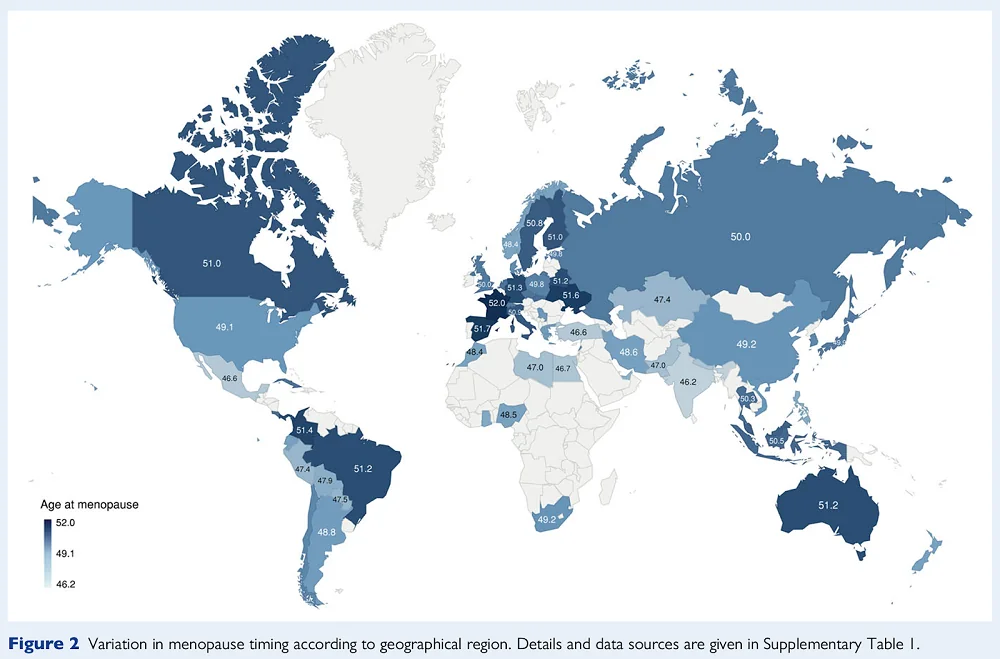
Menopause is tightly linked to female reproductive aging and ovarian reserve. In brief, females are born with a predetermined number of ovarian follicles, which can mature into eggs ready for fertilization. Once ovaries run out of suitable follicles, a woman undergoes menopause [4].
However, some women might experience early menopause (between 41 and 45) or premature menopause at age 40 or earlier. Early menopause can occur naturally if the ovaries stop egg production prematurely, or it can result from chemotherapy or surgical removal of the ovaries.
Premature menopause affects 1 in 250 women under 30 and 1 in 100 women under 40 [1, 5]. Apart from infertility, premature menopause also increases the risk of premature death, neurological diseases, psychosexual dysfunction, mood disorders, osteoporosis, and ischemic heart disease [6].
Factors impacting the timing of menopause
Since age at menopause has a wide range, researchers have studied what impacts its timing, including multiple genetic determinants [7, 8] as well as lifestyle and environmental factors.
Factors that increase early and premature menopause risk are cigarette smoking, being underweight, not giving birth to children (nulliparity), being a multiple-birth child, early age of first menstruation, short and/or very regular menstrual cycles between the ages of 18 to 22 years, and epilepsy [9-14].
Evolutionary theories of menopause
About one-third of the female lifespan is spent after menopause. From an evolutionary point of view, this doesn’t immediately make sense, as post-menopausal women can no longer pass on their genes, which would presumably make selecting for post-menopausal longevity impossible. However, this is what has actually occurred.
A decline in reproductive function with age is observed in different species, from nematodes and insects to birds and mammals [15]. The antagonistic pleiotropy theory offers a possible explanation for this situation, as genes that are advantageous for younger organisms can be harmful later in life [16]. In this case, genes that can help maximize reproduction during the early stages of life can lead to aging.
The relationship between follicular depletion and menopause may be an example of antagonistic pleiotropy. Follicular depletion is important for regulating menstrual cycles at younger reproductive ages; it suggests that selective pressure is not on menopause but on follicular depletion [3]. However, this leaves a question as to why “selection for follicular depletion to maintain regular ovarian cycles led to the evolution of complete reproductive cessation well before the end of potential lifespan in women, but not in the vast majority of other species” [3]. Early menopause is not experienced in most species, including chimpanzees and elephants, whose lifespan is similar to that of humans but do not completely lose reproductive ability with age [17]. Humans and a few species of toothed whales are the only vertebrates whose reproductive and somatic senescence do not occur together [18].
The researchers developed a few hypotheses regarding the evolutionary function of menopause in humans and the lengthy post-reproductive span. Some of the most popular are the mother, the reproductive conflict, and the grandmother hypotheses [3].
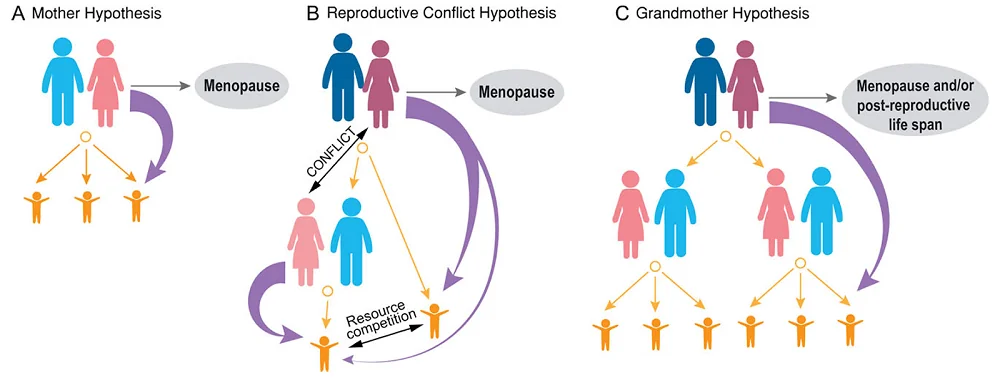
The mother hypothesis is based on the fact that human children require long parental care compared to other species, and the mother plays an essential function in that care. However, if the female is older, her risk of dying in childbirth is higher. Therefore, according to this hypothesis, menopause evolved to avoid that risk and instead ensure that a mother would invest her resources in raising the children she already has until they are independent [16].
The second hypothesis, the reproductive conflict hypothesis, is based on patrilocal societies, in which a married couple resides near the husband’s family. In such a case, the reproductive spans of generations would overlap, creating competition for resources needed for children between young females and their mothers-in-law.
The third hypothesis, the grandmother hypothesis [19], suggests that life following menopause serves as an adaptation to help raise grandchildren. Since, in the past, mothers had multiple offspring under their care, the help of grandmothers, with their expertise and resources, could help the offspring succeed.
All of these concepts have pitfalls, and various studies have offered support to them but suggest that they have shortcomings [9, 20-22].
Hormonal changes
The ovaries are hormone-producing organs. They produce estradiol and progesterone, two hormones essential in regulating ovulation [23].
Estrogen (E2, 17β-estradiol) is a female sex hormone. The onset of menopause leads to a decrease in estrogen levels to the levels of age-matched men [24]. This has profound consequences for the functioning of the entire female body. For example, a growing body of evidence suggests that a menopause-related decrease in estrogen levels increases female susceptibility to neurological issues.
Changes in estrogen levels also impact metabolic processes. Estrogen has a role in raising HDL (“good”) cholesterol, reducing LDL (“bad”) cholesterol, causing vasodilation (widening of blood vessels resulting from relaxation of the blood vessel’s muscular wall), and protecting against osteoporosis [25-27].
The brain can produce estrogen, which has been found to affect both emotion and cognition [28]. It was also described to have neuroprotective properties by modulating many molecular pathways [29-31]. A neuroprotective effect is also suggested by the incidence and mortality rates of stroke, which differ between men and women at different stages of their lives. Before menopause, these rates are lower in women than in men, but after menopause, it is the other way around [28].
Hormone replacement therapy
Since menopause is associated with a drop in estrogen, its symptoms can be addressed by hormone replacement therapy (HRT), which can be offered to peri- or early postmenopausal women. However, HRT is not a one-size-fits-all approach, as the strength and duration of specific menopausal symptoms and the risks of side effects vary from woman to woman. Therefore, HRT is only administered under the guidance of medical professionals.
As part of HRT, a woman with an intact uterus is likely to be prescribed progestogens alongside estrogen. The role of progestogens is to protect the endometrium from the excessive proliferation of cells (endometrial hyperplasia) and endometrial cancer [32, 33].
Both progestogen and estrogen can be delivered in different ways, including orally, transdermally (patch, gel, or spray), and subcutaneous implants, with various chemical formulations, doses, and schedules. Each of those approaches has distinct advantages and side effects [34-36].
Cognitive decline
Menopause and cognitive decline are linked as early as the peri-menopausal period, when some women can experience mild cognitive impairment [1]. As the menopausal transition progresses, cognitive impairment seems to affect even 70% of postmenopausal women [1], with memory loss being the most frequently reported [37].
Studies suggest that mild cognitive impairment can be preceded by menopause-related subjective memory impairment. This subjective cognitive decline is linked to later-life Alzheimer’s disease [38]. Some research has found that Alzheimer’s disease has a three-stage manifestation: subjective memory impairment, then mild cognitive impairment, and finally dementia [39].
While all older women will experience menopause, not all will develop Alzheimer’s disease nor suffer from cognitive decline. More research is needed to address who is the most at risk. Still, some factors that impact the risk of developing Alzheimer’s include sex hormone levels, genes, comorbid diseases (such as cardiovascular disease), and the environment [28].
The researchers believe that there is a relationship between cognitive decline symptoms and stress and depression present in the initial transition into menopause [40]. Whether depression contributes to memory dysfunction or vice versa is still debated. However, there is some emerging evidence to support the claim that depression might be the first sign of cognitive decline [41].
The increased risk of depression during the early stages of menopause provides a link between menopause-related estrogen loss and neurological changes [40]. HRT during perimenopause can significantly reduce depression risk [42-44]. The timing of estrogen therapy seems to be essential, since postmenopausal women do not seem to benefit from this treatment [45, 46].
Surgically induced menopause, such as through ovarian removal (oophorectomy), also increases the risk of depression and dementia [47, 48].
Not all women who experience mild cognitive decline have dementia afterward, and their cognitive abilities can also improve over time [1].
Estrogen replacement therapy seems to impact menopausal and postmenopausal women’s cognitive abilities. Multiple papers addressing the relationship between HRT and dementia, conducted in the 1990s, showed a decreased risk of dementia and Alzheimer’s disease in women undergoing HRT [49-55]. This prompted significant interest and more studies addressing this relationship.
One of the studies sparked controversy, as it contradicted earlier results. This was the Women’s Health Initiative Memory Study (WHIMS), a randomized, double-blind, placebo-controlled clinical trial that recruited 4,532 women. The authors concluded: “Estrogen plus progestin therapy increased the risk for probable dementia in postmenopausal women aged 65 years or older” [56]. Two decades later, this study still makes the relationship between hormone therapy and dementia risk uncertain [57].
Researchers have quested to explain these contradicting results. One popular explanation is referred to as the critical window hypothesis. This hypothesis suggests that HRT, to be effective, needs to be administered during the ‘critical window’ shortly after the menopausal transition [58, 59].
A wide range of studies has been done to address this hypothesis. Additionally, data from previously conducted studies was reanalyzed based on the relationship between age and HRT treatment. Multiple studies found that women who started HRT closer to their age at menopause had a lower risk of Alzheimer’s disease and dementia and improved cognitive performance [60-64].
However, there is also plenty of evidence that opposes the critical window hypothesis. For example, some studies do not show improvement in cognition in recently postmenopausal women on HRT [65, 66]. Other studies show improvements in cognition among older women receiving HRT [67, 68]. There are also studies that show no impact of HRT on cognition [69, 70].
Such variability in results suggests that other, yet-to-be-discovered, variables play a role in the success or failure of HRT in postmenopausal women.
Cardiovascular health
Postmenopausal women have a greater incidence of cardiovascular problems than premenopausal women do [71]. Multiple studies also report that early menopause, compared to menopause in the mid-forties or fifties, is linked to a 30-50% higher risk of cardiovascular diseases [72, 73].
Research suggests that the link between cardiovascular diseases and early menopause might be bidirectional [74]. Pooled data of over 170,000 women from nine studies suggests a twofold increase in the risk of early menopause (before 45) if a female experiences her first cardiovascular disease event before 35 years of age, compared to women without any premenopausal cardiovascular disease events [75].
Studies have also explored the risk of cardiometabolic diseases following surgical menopause (without estrogen therapy use), suggesting higher cardiometabolic disease risks in women who experienced surgical menopause compared to natural menopause. Some of those studies suggest that estrogen-replacement therapy might help to mitigate the risk [76. 77].
HRT and its impact on cardiovascular health were also investigated in women undergoing natural menopause. As part of the Women’s Health Initiative Randomized Trials (WHIRT), 27,347 postmenopausal women were followed up on for 18 years. A study using this data found that neither estrogen-only nor combined progestogen therapy led to an increased risk of cardiovascular-related mortality [78].
Similarly to cognitive health, cardiovascular health seems to be impacted by the timing of HRT treatment. The window of opportunity of HRT, with or without progestogen, for cardiovascular health has been supported by several studies, including some randomized controlled trials [32, 79, 80].
Musculoskeletal health
Musculoskeletal function problems, such as joint stiffness, musculoskeletal pain, intervertebral disc thinning, osteoarthritic joint changes, and loss of muscle mass (sarcopenia) are exacerbated by menopause, and many of them are linked to menopause-related estrogen loss. While lifestyle interventions such as diet, exercise, or weight optimization can help manage the symptoms of those conditions, HRT can also be an option for some of them [81-83].
An analysis of the Women’s Health Initiative randomized trial of 10,739 postmenopausal women who have had a hysterectomy and who received estrogen-alone therapy showed “modest but sustained reduction in the frequency of joint pain” [84].
However, there is no consensus regarding HRT’s effect on muscle strength in menopausal women. A meta-analysis of 23 studies of postmenopausal women who took estrogen-based hormone therapy found a roughly 5% improvement in strength comparred to a control group [85]. On the other hand, a more recent meta-analysis of 12 studies of 4474 postmenopausal women aged 50 years and older undergoing either estrogen-progesterone combination HRT or estrogen-only HRT concluded that the results “did not show a significant beneficial or detrimental association of HRT with muscle mass” [86].
However, HRT showed effectiveness in preserving bone density, preventing osteoporosis, and reducing the risk of osteoporosis-related fractures. Osteoporosis is a skeletal disorder that leads to bone mass reduction and deterioration of bone tissue, leading to increased fracture risk. Menopause-related estrogen loss contributes to the acceleration of bone density loss [32].
A review of 28 studies that included 33,426 participants concluded that HRT was associated with a reduced risk of total, hip, and vertebral fractures. However, the reviewers suggested that the effectiveness of HRT is reduced at the cessation of treatment, or when it is started after 60 years of age [87]. However, there are also studies suggesting that HRT has a protective effect against bone loss and osteoporotic fracture even after it is discontinued [88].
HRT in managing other menopausal symptoms
Menopause is associated with many symptoms that lower the quality of life. HRT can be used to address many of those symptoms. Some of the most common menopause symptoms are vasomotor symptoms, such as hot flashes and night sweats, which can lead to sleep disturbances, increased tiredness, anxiety, and depressed mood and are associated with palpitations [32].
A study that reviewed 24 double-blind, randomized, placebo-controlled trials that included over 3,000 participants concluded that using oral hormone therapy, compared to placebo, was “highly effective in alleviating hot flushes and night sweats.” However, there is still a need to analyze differences between hormonal doses, product types, or regimens [89].
Menopause might also be associated with decreased sexual desire and arousal. In such cases, leading international menopausal societies recommend using testosterone therapy at levels within the female physiological range [90].
Problems with sexual dysfunction can also arise from other menopause-related symptoms such as painful sexual intercourse or urethral syndrome, a painful or burning sensation during urination, urinary urgency, and frequency. Those results result from vaginal, lower urinary tract, and pelvic floor tissue atrophy caused by the menopause-related loss of collagen. To remedy this, topical vaginal estrogen therapy is often recommended. Estrogen positively impacts blood supply to those tissues, vaginal lubrication, and restoration of acidic pH [32, 91, 92].
Risks associated with HRT
One of the main reasons for concern about HRT is the risk of breast cancer. Recently published recommendations regarding hormone therapy in menopausal women cite literature that suggests that this risk may depend on the therapy used [32, 93, 94].
For example, the literature suggests that estrogen and progestogen HRT is associated with an increased risk of breast cancer, but compared to placebo, they have no significant difference in breast cancer mortality. When estrogen is used alone, different studies show either a slight increase, no increase, or a decrease in the risk of breast cancer and a significant reduction in breast cancer mortality compared with placebo [95, 96].
There are also reports suggesting a link between stroke and HRT. For example, data from the WHIRT study showed an increased risk of stroke among women taking estrogen, whether or not it was combined with progestogen [87].
Another review of 19 randomized clinical trials of 40,410 post-menopausal women reported an increased risk of stroke among the women who started treatment more than 10 years after menopause but not the women who started hormone therapy less than 10 years after menopause [80].
The dose and route of the administration seem to matter, as one of the comparative analyses of stroke risk found that low doses of estrogen delivered by patch did not increase stroke risk compared to controls. However, the risk was increased for women using high-dose patches and women who took hormones orally [98].
The method of administration also significantly affects the risk of blood clots in veins (venous thromboembolisms). In an observational study of 80,396 women aged 40-79 with a primary diagnosis of venous thromboembolism and 391,494 female controls, a transdermal method of HRT administration was not associated with this condition. The same study reported that oral therapy, when compared to controls, was associated with a significantly increased risk of venous thromboembolism. This risk was increased whether or not the estrogen was part of a combined preparation [99].
Similar results were obtained in different studies, with some reporting higher risks among users of combined estrogen and progestogen compared to estrogen-only therapy and differences among different pharmacological classes of progestogens in venous thromboembolism risk [100].
Conclusions
The research into menopausal transition has many unexplored areas and unanswered questions. Years of research focused only on male model organisms contributed to many gaps in the knowledge of female biology that need to be addressed in the future to give women well-informed recommendations regarding treatments and risks associated with them.
Literature
[1] Sochocka, M., Karska, J., Pszczołowska, M., Ochnik, M., Fułek, M., Fułek, K., Kurpas, D., Chojdak-Łukasiewicz, J., Rosner-Tenerowicz, A., & Leszek, J. (2023). Cognitive decline in early and premature menopause. International Journal of Molecular Sciences, 24(7).
[2] Soules, M. R., Sherman, S., Parrott, E., Rebar, R., Santoro, N., Utian, W., & Woods, N. (2001). Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertility and Sterility, 76(5), 874–878.
[3] Laisk, T., Tšuiko, O., Jatsenko, T., Hõrak, P., Otala, M., Lahdenperä, M., Lummaa, V., Tuuri, T., Salumets, A., & Tapanainen, J. S. (2019). Demographic and evolutionary trends in ovarian function and aging. Human Reproduction Update, 25(1), 34–50.
[4] Santoro, N., Roeca, C., Peters, B. A., & Neal-Perry, G. (2021). The menopause transition: Signs, symptoms, and management options. The Journal of Clinical Endocrinology and Metabolism, 106(1), 1–15.
[5] Luborsky, J. L., Meyer, P., Sowers, M. F., Gold, E. B., & Santoro, N. (2003). Premature menopause in a multi-ethnic population study of the menopause transition. Human Reproduction, 18(1), 199–206.
[6] Okeke, T., Anyaehie, U., & Ezenyeaku, C. (2013). Premature menopause. Annals of Medical and Health Sciences Research, 3(1), 90–95.
[7] Laven, J. S. E. (2016). Primary Ovarian Insufficiency. Seminars in Reproductive Medicine, 34(4), 230–234.
[8] Ruth, K. S., Day, F. R., Hussain, J., Martínez-Marchal, A., Aiken, C. E., Azad, A., Thompson, D. J., Knoblochova, L., Abe, H., Tarry-Adkins, J. L., Gonzalez, J. M., Fontanillas, P., Claringbould, A., Bakker, O. B., Sulem, P., Walters, R. G., Terao, C., Turon, S., Horikoshi, M., … et al. (2021). Genetic insights into biological mechanisms governing human ovarian ageing. Nature, 596(7872), 393–397.
[9] Yasui, T., Hayashi, K., Mizunuma, H., Kubota, T., Aso, T., Matsumura, Y., Lee, J.-S., & Suzuki, S. (2012). Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas, 72(3), 249–255.
[10] Whitcomb, B. W., Purdue-Smithe, A. C., Szegda, K. L., Boutot, M. E., Hankinson, S. E., Manson, J. E., Rosner, B., Willett, W. C., Eliassen, A. H., & Bertone-Johnson, E. R. (2018). Cigarette smoking and risk of early natural menopause. American Journal of Epidemiology, 187(4), 696–704.
[11] Sveinsson, O., & Tomson, T. (2014). Epilepsy and menopause: potential implications for pharmacotherapy. Drugs & Aging, 31(9), 671–675.
[12] Mishra, G. D., Chung, H.-F., Cano, A., Chedraui, P., Goulis, D. G., Lopes, P., Mueck, A., Rees, M., Senturk, L. M., Simoncini, T., Stevenson, J. C., Stute, P., Tuomikoski, P., & Lambrinoudaki, I. (2019). EMAS position statement: Predictors of premature and early natural menopause. Maturitas, 123, 82–88.
[13] Mishra, G. D., Pandeya, N., Dobson, A. J., Chung, H.-F., Anderson, D., Kuh, D., Sandin, S., Giles, G. G., Bruinsma, F., Hayashi, K., Lee, J. S., Mizunuma, H., Cade, J. E., Burley, V., Greenwood, D. C., Goodman, A., Simonsen, M. K., Adami, H.-O., Demakakos, P., & Weiderpass, E. (2017). Early menarche, nulliparity and the risk for premature and early natural menopause. Human Reproduction, 32(3), 679–686.
[14] Whitcomb, B. W., Purdue-Smithe, A., Hankinson, S. E., Manson, J. E., Rosner, B. A., & Bertone-Johnson, E. R. (2018). Menstrual cycle characteristics in adolescence and early adulthood are associated with risk of early natural menopause. The Journal of Clinical Endocrinology and Metabolism, 103(10), 3909–3918.
[15] Jones, O. R., Scheuerlein, A., Salguero-Gómez, R., Camarda, C. G., Schaible, R., Casper, B. B., Dahlgren, J. P., Ehrlén, J., García, M. B., Menges, E. S., Quintana-Ascencio, P. F., Caswell, H., Baudisch, A., & Vaupel, J. W. (2014). Diversity of ageing across the tree of life. Nature, 505(7482), 169–173.
[16] Williams, G. C. (1957). Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution, 11(4), 398.
[17] Lahdenperä, M., Mar, K. U., & Lummaa, V. (2014). Reproductive cessation and post-reproductive lifespan in Asian elephants and pre-industrial humans. Frontiers in Zoology, 11, 54.
[18] Ellis, S., Franks, D. W., Nattrass, S., Cant, M. A., Bradley, D. L., Giles, D., Balcomb, K. C., & Croft, D. P. (2018). Postreproductive lifespans are rare in mammals. Ecology and Evolution, 8(5), 2482–2494.
[19] Hawkes, K., O’Connell, J. F., Jones, N. G., Alvarez, H., & Charnov, E. L. (1998). Grandmothering, menopause, and the evolution of human life histories. Proceedings of the National Academy of Sciences of the United States of America, 95(3), 1336–1339.
[20] Snopkowski, K., Moya, C., & Sear, R. (2014). A test of the intergenerational conflict model in Indonesia shows no evidence of earlier menopause in female-dispersing groups. Proceedings. Biological Sciences / the Royal Society, 281(1788), 20140580.
[21] Lahdenperä, M., Lummaa, V., Helle, S., Tremblay, M., & Russell, A. F. (2004). Fitness benefits of prolonged post-reproductive lifespan in women. Nature, 428(6979), 178–181.
[22] Lahdenperä, M., Gillespie, D. O. S., Lummaa, V., & Russell, A. F. (2012). Severe intergenerational reproductive conflict and the evolution of menopause. Ecology Letters, 15(11), 1283–1290.x
[23] Holesh, J. E., Bass, A. N., & Lord, M. (2024). Physiology, Ovulation. In StatPearls. StatPearls Publishing.
[24] Iqbal, J., & Zaidi, M. (2009). Understanding estrogen action during menopause. Endocrinology, 150(8), 3443–3445.
[25] Wang, H. H., Liu, M., Clegg, D. J., Portincasa, P., & Wang, D. Q.-H. (2009). New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochimica et Biophysica Acta, 1791(11), 1037–1047.
[26] Barton, M., Meyer, M. R., & Prossnitz, E. R. (2013). Alike but not the same: anatomic heterogeneity of estrogen receptor-mediated vasodilation. Journal of Cardiovascular Pharmacology, 62(1), 22–25.
[27] Kelsey, J. L. (1989). Risk factors for osteoporosis and associated fractures. Public Health Reports (Washington, D.C. : 1974), 104 Suppl, 14–20.
[28] Maioli, S., Leander, K., Nilsson, P., & Nalvarte, I. (2021). Estrogen receptors and the aging brain. Essays in Biochemistry, 65(6), 913–925.
[29] Céspedes Rubio, Á. E., Pérez-Alvarez, M. J., Lapuente Chala, C., & Wandosell, F. (2018). Sex steroid hormones as neuroprotective elements in ischemia models. The Journal of Endocrinology, 237(2), R65–R81.
[30] Neuroprotection of sex steroids – Minerva Endocrinologica 2010 June;35(2):127-43 – Minerva Medica – Journals. (n.d.). Retrieved January 1, 2025, from https://www.minervamedica.it/en/journals/minerva-endocrinology/article.php?cod=R07Y2010N02A0127
[31] Lan, Y.-L., Zhao, J., & Li, S. (2014). Estrogen receptors’ neuroprotective effect against glutamate-induced neurotoxicity. Neurological Sciences, 35(11), 1657–1662.
[32] Vigneswaran, K., & Hamoda, H. (2022). Hormone replacement therapy – Current recommendations. Best Practice & Research. Clinical Obstetrics & Gynaecology, 81, 8–21.
[33] Furness, S., Roberts, H., Marjoribanks, J., Lethaby, A., Hickey, M., & Farquhar, C. (2009). Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database of Systematic Reviews, 2, CD000402.
[34] Bagot, C. N., Marsh, M. S., Whitehead, M., Sherwood, R., Roberts, L., Patel, R. K., & Arya, R. (2010). The effect of estrone on thrombin generation may explain the different thrombotic risk between oral and transdermal hormone replacement therapy. Journal of Thrombosis and Haemostasis, 8(8), 1736–1744.
[35] Canonico, M., Fournier, A., Carcaillon, L., Olié, V., Plu-Bureau, G., Oger, E., Mesrine, S., Boutron-Ruault, M.-C., Clavel-Chapelon, F., & Scarabin, P.-Y. (2010). Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: results from the E3N cohort study. Arteriosclerosis, Thrombosis, and Vascular Biology, 30(2), 340–345.
[36] Stute, P., Wildt, L., & Neulen, J. (2018). The impact of micronized progesterone on breast cancer risk: a systematic review. Climacteric, 21(2), 111–122.
[37] Betti, S., Orsini, M. R., Sciaky, R., Cristini, C., Cesa-Bianchi, G., & Zandonini, G. F. (2001). Attitudes towards menopause in a group of women followed in a public service for menopause counseling. Aging (Milan, Italy), 13(4), 331–338.
[38] Reuben, R., Karkaby, L., McNamee, C., Phillips, N. A., & Einstein, G. (2021). Menopause and cognitive complaints: are ovarian hormones linked with subjective cognitive decline? Climacteric, 24(4), 321–332.
[39] Jessen, F., Wiese, B., Bachmann, C., Eifflaender-Gorfer, S., Haller, F., Kölsch, H., Luck, T., Mösch, E., van den Bussche, H., Wagner, M., Wollny, A., Zimmermann, T., Pentzek, M., Riedel-Heller, S. G., Romberg, H.-P., Weyerer, S., Kaduszkiewicz, H., Maier, W., Bickel, H., & German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. (2010). Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Archives of General Psychiatry, 67(4), 414–422.
[40] Morrison, J. H., Brinton, R. D., Schmidt, P. J., & Gore, A. C. (2006). Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. The Journal of Neuroscience, 26(41), 10332–10348.
[41] Geerlings, M. I., Schoevers, R. A., Beekman, A. T., Jonker, C., Deeg, D. J., Schmand, B., Adèr, H. J., Bouter, L. M., & Van Tilburg, W. (2000). Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. The British Journal of Psychiatry, 176, 568–575.
[42] Dwyer, J. B., Aftab, A., Radhakrishnan, R., Widge, A., Rodriguez, C. I., Carpenter, L. L., Nemeroff, C. B., McDonald, W. M., Kalin, N. H., & APA Council of Research Task Force on Novel Biomarkers and Treatments. (2020). Hormonal treatments for major depressive disorder: state of the art. The American Journal of Psychiatry, 177(8), 686–705.
[43] Soares, C. N., Almeida, O. P., Joffe, H., & Cohen, L. S. (2001). Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry, 58(6), 529–534.
[44] Schmidt, P. J., Nieman, L., Danaceau, M. A., Tobin, M. B., Roca, C. A., Murphy, J. H., & Rubinow, D. R. (2000). Estrogen replacement in perimenopause-related depression: a preliminary report. American Journal of Obstetrics and Gynecology, 183(2), 414–420.
[45] Morrison, M. F., Kallan, M. J., Ten Have, T., Katz, I., Tweedy, K., & Battistini, M. (2004). Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biological Psychiatry, 55(4), 406–412.
[46] Guo, H., Liu, M., Zhang, L., Wang, L., Hou, W., Ma, Y., & Ma, Y. (2020). The critical period for neuroprotection by estrogen replacement therapy and the potential underlying mechanisms. Current Neuropharmacology, 18(6), 485–500.
[47] Parker, W. H. (2010). Bilateral oophorectomy versus ovarian conservation: effects on long-term women’s health. Journal of Minimally Invasive Gynecology, 17(2), 161–166.
[48] Rocca, W. A., Grossardt, B. R., Geda, Y. E., Gostout, B. S., Bower, J. H., Maraganore, D. M., de Andrade, M., & Melton, L. J. (2008). Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause, 15(6), 1050–1059.
[49] Mortel, K. F., & Meyer, J. S. (1995). Lack of postmenopausal estrogen replacement therapy and the risk of dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 7(3), 334–337.
[50] Tang, M. X., Jacobs, D., Stern, Y., Marder, K., Schofield, P., Gurland, B., Andrews, H., & Mayeux, R. (1996). Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. The Lancet, 348(9025), 429–432.
[51] Kawas, C., Resnick, S., Morrison, A., Brookmeyer, R., Corrada, M., Zonderman, A., Bacal, C., Lingle, D. D., & Metter, E. (1997). A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology, 48(6), 1517–1521.
[52] Baldereschi, M., Di Carlo, A., Lepore, V., Bracco, L., Maggi, S., Grigoletto, F., Scarlato, G., & Amaducci, L. (1998). Estrogen-replacement therapy and Alzheimer’s disease in the Italian Longitudinal Study on Aging. Neurology, 50(4), 996–1002.
[53] Slooter, A. J., Bronzova, J., Witteman, J. C., Van Broeckhoven, C., Hofman, A., & van Duijn, C. M. (1999). Estrogen use and early onset Alzheimer’s disease: a population-based study. Journal of Neurology, Neurosurgery, and Psychiatry, 67(6), 779–781.
[54] Waring, S. C., Rocca, W. A., Petersen, R. C., O’Brien, P. C., Tangalos, E. G., & Kokmen, E. (1999). Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology, 52(5), 965–970.
[55] Zandi, P. P., Carlson, M. C., Plassman, B. L., Welsh-Bohmer, K. A., Mayer, L. S., Steffens, D. C., Breitner, J. C. S., & Cache County Memory Study Investigators. (2002). Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. The Journal of the American Medical Association, 288(17), 2123–2129.
[56] Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., Hendrix, S. L., Jones, B. N., Assaf, A. R., Jackson, R. D., Kotchen, J. M., Wassertheil-Smoller, S., Wactawski-Wende, J., & WHIMS Investigators. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. The Journal of the American Medical Association, 289(20), 2651–2662.
[57] Mills, Z. B., Faull, R. L. M., & Kwakowsky, A. (2023). Is hormone replacement therapy a risk factor or a therapeutic option for alzheimer’s disease? International Journal of Molecular Sciences, 24(4).
[58] Maki, P. M. (2006). Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience, 138(3), 1027–1030.
[59] Sherwin, B. B. (2007). The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? Journal of Neuroendocrinology, 19(2), 77–81.
[60] Shao, H., Breitner, J. C. S., Whitmer, R. A., Wang, J., Hayden, K., Wengreen, H., Corcoran, C., Tschanz, J., Norton, M., Munger, R., Welsh-Bohmer, K., Zandi, P. P., & Cache County Investigators. (2012). Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology, 79(18), 1846–1852.
[61] Henderson, V. W., Benke, K. S., Green, R. C., Cupples, L. A., Farrer, L. A., & MIRAGE Study Group. (2005). Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. Journal of Neurology, Neurosurgery, and Psychiatry, 76(1), 103–105.
[62] Whitmer, R. A., Quesenberry, C. P., Zhou, J., & Yaffe, K. (2011). Timing of hormone therapy and dementia: the critical window theory revisited. Annals of Neurology, 69(1), 163–169.
[63] Greendale, G. A., Huang, M. H., Wight, R. G., Seeman, T., Luetters, C., Avis, N. E., Johnston, J., & Karlamangla, A. S. (2009). Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology, 72(21), 1850–1857.
[64] MacLennan, A. H., Henderson, V. W., Paine, B. J., Mathias, J., Ramsay, E. N., Ryan, P., Stocks, N. P., & Taylor, A. W. (2006). Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause, 13(1), 28–36.
[65] Maki, P. M., Gast, M. J., Vieweg, A. J., Burriss, S. W., & Yaffe, K. (2007). Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology, 69(13), 1322–1330.
[66] LeBlanc, E. S., Neiss, M. B., Carello, P. E., Samuels, M. H., & Janowsky, J. S. (2007). Hot flashes and estrogen therapy do not influence cognition in early menopausal women. Menopause, 14(2), 191–202.
[67] Wolf, O. T., Kudielka, B. M., Hellhammer, D. H., Törber, S., McEwen, B. S., & Kirschbaum, C. (1999). Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology, 24(7), 727–741.
[68] Alhola, P., Tuomisto, H., Saarinen, R., Portin, R., Kalleinen, N., & Polo-Kantola, P. (2010). Estrogen + progestin therapy and cognition: a randomized placebo-controlled double-blind study. The Journal of Obstetrics and Gynaecology Research, 36(4), 796–802.
[69] Pefanco, M. A., Kenny, A. M., Kaplan, R. F., Kuchel, G., Walsh, S., Kleppinger, A., & Prestwood, K. (2007). The effect of 3-year treatment with 0.25 mg/day of micronized 17beta-estradiol on cognitive function in older postmenopausal women. Journal of the American Geriatrics Society, 55(3), 426–431.
[70] Henderson, V. W., St John, J. A., Hodis, H. N., McCleary, C. A., Stanczyk, F. Z., Shoupe, D., Kono, N., Dustin, L., Allayee, H., & Mack, W. J. (2016). Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology, 87(7), 699–708.
[71] Kannel, W. B., Hjortland, M. C., McNamara, P. M., & Gordon, T. (1976). Menopause and risk of cardiovascular disease: the Framingham study. Annals of Internal Medicine, 85(4), 447–452.
[72] Zhu, D., Chung, H.-F., Dobson, A. J., Pandeya, N., Giles, G. G., Bruinsma, F., Brunner, E. J., Kuh, D., Hardy, R., Avis, N. E., Gold, E. B., Derby, C. A., Matthews, K. A., Cade, J. E., Greenwood, D. C., Demakakos, P., Brown, D. E., Sievert, L. L., Anderson, D., … Mishra, G. D. (2019). Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. The Lancet. Public Health, 4(11), e553–e564.
[73] Muka, T., Oliver-Williams, C., Kunutsor, S., Laven, J. S. E., Fauser, B. C. J. M., Chowdhury, R., Kavousi, M., & Franco, O. H. (2016). Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiology, 1(7), 767–776.
[74] Roa-Díaz, Z. M., Raguindin, P. F., Bano, A., Laine, J. E., Muka, T., & Glisic, M. (2021). Menopause and cardiometabolic diseases: What we (don’t) know and why it matters. Maturitas, 152, 48–56.
[75] Zhu, D., Chung, H.-F., Pandeya, N., Dobson, A. J., Hardy, R., Kuh, D., Brunner, E. J., Bruinsma, F., Giles, G. G., Demakakos, P., Lee, J. S., Mizunuma, H., Hayashi, K., Adami, H.-O., Weiderpass, E., & Mishra, G. D. (2019). Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. European Journal of Epidemiology, 34(3), 235–246.
[76] Lobo, R. A. (2007). Surgical menopause and cardiovascular risks. Menopause, 14(3 Pt 2), 562–566.
[77] Colditz, G. A., Willett, W. C., Stampfer, M. J., Rosner, B., Speizer, F. E., & Hennekens, C. H. (1987). Menopause and the risk of coronary heart disease in women. The New England Journal of Medicine, 316(18), 1105–1110.
[78] Manson, J. E., Aragaki, A. K., Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., Chlebowski, R. T., Howard, B. V., Thomson, C. A., Margolis, K. L., Lewis, C. E., Stefanick, M. L., Jackson, R. D., Johnson, K. C., Martin, L. W., Shumaker, S. A., Espeland, M. A., Wactawski-Wende, J., & WHI Investigators. (2017). Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. The Journal of the American Medical Association, 318(10), 927–938.
[79] Hodis, H. N., Mack, W. J., Henderson, V. W., Shoupe, D., Budoff, M. J., Hwang-Levine, J., Li, Y., Feng, M., Dustin, L., Kono, N., Stanczyk, F. Z., Selzer, R. H., Azen, S. P., & ELITE Research Group. (2016). Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. The New England Journal of Medicine, 374(13), 1221–1231.
[80] Boardman, H. M. P., Hartley, L., Eisinga, A., Main, C., Roqué i Figuls, M., Bonfill Cosp, X., Gabriel Sanchez, R., & Knight, B. (2015). Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database of Systematic Reviews, 2015(3), CD002229.
[81] Magliano, M. (2010). Menopausal arthralgia: Fact or fiction. Maturitas, 67(1), 29–33.
[82] Cho, E.-J., Choi, Y., Jung, S.-J., & Kwak, H.-B. (2022). Role of exercise in estrogen deficiency-induced sarcopenia. Journal of Exercise Rehabilitation, 18(1), 2–9.
[83] Howe, T. E., Shea, B., Dawson, L. J., Downie, F., Murray, A., Ross, C., Harbour, R. T., Caldwell, L. M., & Creed, G. (2011). Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database of Systematic Reviews, 7, CD000333.
[84] Chlebowski, R. T., Cirillo, D. J., Eaton, C. B., Stefanick, M. L., Pettinger, M., Carbone, L. D., Johnson, K. C., Simon, M. S., Woods, N. F., & Wactawski-Wende, J. (2013). Estrogen alone and joint symptoms in the Women’s Health Initiative randomized trial. Menopause, 20(6), 600–608.
[85] Greising, S. M., Baltgalvis, K. A., Lowe, D. A., & Warren, G. L. (2009). Hormone therapy and skeletal muscle strength: a meta-analysis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64(10), 1071–1081.
[86] Javed, A. A., Mayhew, A. J., Shea, A. K., & Raina, P. (2019). Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Network Open, 2(8), e1910154.
[87] Zhu, L., Jiang, X., Sun, Y., & Shu, W. (2016). Effect of hormone therapy on the risk of bone fractures: a systematic review and meta-analysis of randomized controlled trials. Menopause, 23(4), 461–470.
[88] Bagger, Y. Z., Tankó, L. B., Alexandersen, P., Hansen, H. B., Møllgaard, A., Ravn, P., Qvist, P., Kanis, J. A., & Christiansen, C. (2004). Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone, 34(4), 728–735.
[89] Maclennan, A. H., Broadbent, J. L., Lester, S., & Moore, V. (2004). Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database of Systematic Reviews, 2004(4), CD002978.
[90] Davis, S. R., Baber, R., Panay, N., Bitzer, J., Cerdas Perez, S., Islam, R. M., Kaunitz, A. M., Kingsberg, S. A., Lambrinoudaki, I., Liu, J., Parish, S. J., Pinkerton, J., Rymer, J., Simon, J. A., Vignozzi, L., & Wierman, M. E. (2019). Global consensus position statement on the use of testosterone therapy for women. Climacteric, 22(5), 429–434.
[91] Pitkin, J., & British Menopause Society medical advisory council. (2018). BMS – Consensus statement. Post Reproductive Health, 24(3), 133–138.
[92] Goldstein, I. (2010). Recognizing and treating urogenital atrophy in postmenopausal women. Journal of Women’s Health, 19(3), 425–432.
[93] Fournier, A., Berrino, F., & Clavel-Chapelon, F. (2008). Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Research and Treatment, 107(1), 103–111.
[94] Collaborative Group on Hormonal Factors in Breast Cancer. (2019). Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet, 394(10204), 1159–1168.
[95] NICE Guideline – British Menopause Society. (n.d.). Retrieved January 9, 2025, from https://thebms.org.uk/publications/nice-guideline/
[96] Chlebowski, R. T., Anderson, G. L., Aragaki, A. K., Manson, J. E., Stefanick, M. L., Pan, K., Barrington, W., Kuller, L. H., Simon, M. S., Lane, D., Johnson, K. C., Rohan, T. E., Gass, M. L. S., Cauley, J. A., Paskett, E. D., Sattari, M., & Prentice, R. L. (2020). Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. The Journal of the American Medical Association, 324(4), 369–380.
[97] Manson, J. E., Chlebowski, R. T., Stefanick, M. L., Aragaki, A. K., Rossouw, J. E., Prentice, R. L., Anderson, G., Howard, B. V., Thomson, C. A., LaCroix, A. Z., Wactawski-Wende, J., Jackson, R. D., Limacher, M., Margolis, K. L., Wassertheil-Smoller, S., Beresford, S. A., Cauley, J. A., Eaton, C. B., Gass, M., … Wallace, R. B. (2013). Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. The Journal of the American Medical Association, 310(13), 1353–1368.
[98] Renoux, C., Dell’aniello, S., Garbe, E., & Suissa, S. (2010). Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ (Clinical Research Ed.), 340, c2519.
[99] Vinogradova, Y., Coupland, C., & Hippisley-Cox, J. (2019). Use of hormone replacement therapy and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases. BMJ (Clinical Research Ed.), 364, k4810.
[100] Canonico, M., Plu-Bureau, G., & Scarabin, P.-Y. (2011). Progestogens and venous thromboembolism among postmenopausal women using hormone therapy. Maturitas, 70(4), 354–360.

