Astrocytes Become Neural Stem Cells After Injury

A new study published in Cell Stem Cell has shown how astrocytes, glial cells that perform multiple critical functions in the brain, can become neural stem cells (NSCs) in response to injury.
Astrocytes’ normal functions
The term ‘astrocyte’, meaning star-shaped cell, refers to a very heterogenous population of cells that perform maintenance, management and constructive functions in the brain, including the formation of synapses between neurons and the maintenance of the blood-brain barrier. The heterogeneity of these cells is such that they are not the same everywhere in the brain, and they have different capabilities and functions depending on their location.
This new study shows that, in addition to these upkeep functions, some astrocytes are capable of transforming into NSCs, providing the mammalian brain with an existent, if limited, capability of recovering from injury.
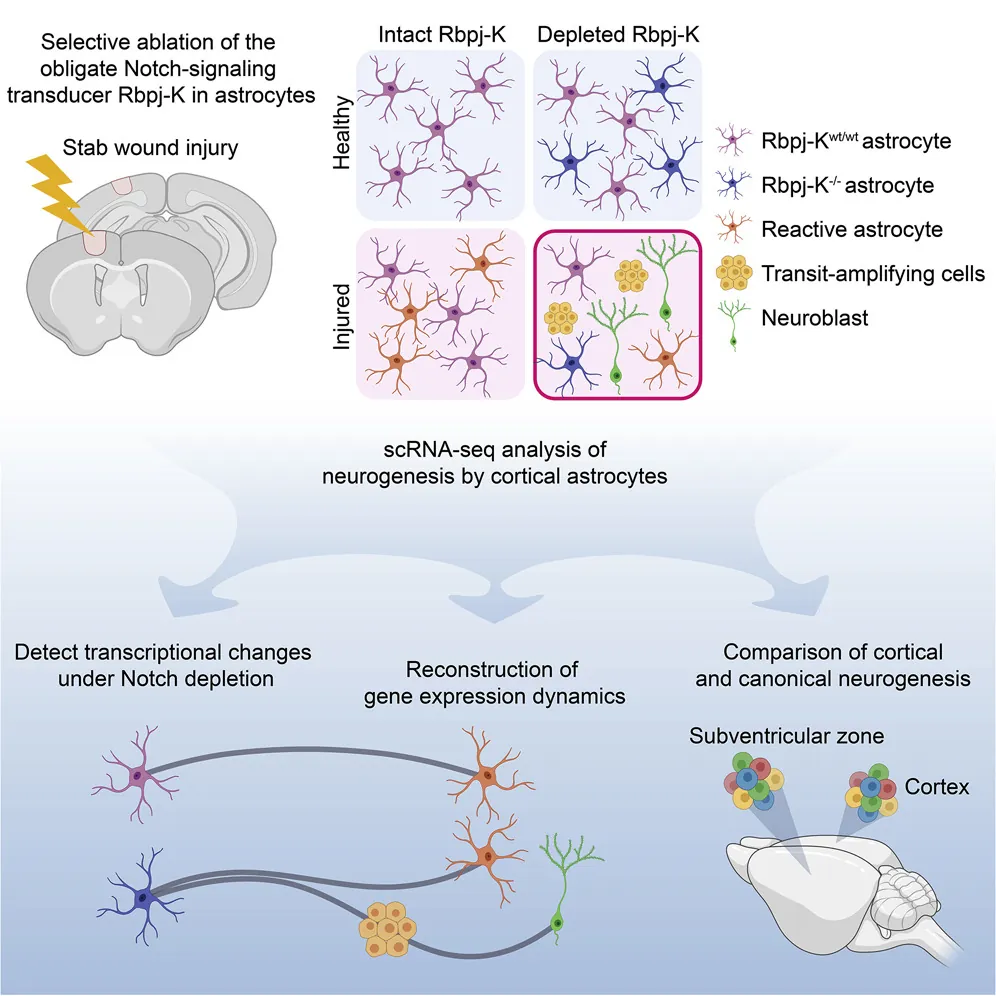
Astrocytes as precursors to NSCs
This study expands on a previous study from 2014, which showed the potential of these cells to become NSCs following a stroke [1]. It details the effects of the protein Notch, which suppresses astrocytes from becoming NSCs, and explains many of the genes that are activated in the absence of this protein. Therefore, the researchers consider certain astrocytes to be neural stem cells in and of themselves.
Alignment of the transcriptional events driving neuronal specification in the cortex and the neurogenic niche further supports the idea that parenchymal astrocytes may be viewed as dormant NSCs that, with the appropriate molecular cues, can be recruited to generate neurons following a trajectory similar to canonical neurogenesis.
It has been hypothesized that parenchymal astrocytes may be considered dormant NSCs because of shared expression of several biomarkers. The present study further supports this notion by showing that, when recruited into the neurogenic program, the transcriptional behavior of committed glia parallels that of adult NSCs from the germinal niche.
Conclusion
Of course, this is a mouse study, and, even though it greatly explains many of the fundamental biochemical processes that allow astrocytes to become neurons, the full relationship between injury and neurogenesis remains unclear, and there are still many questions that must be answered before this research could be used to develop a therapy. However, the researchers are aware of these limitations, and they have helpfully made their important data public in order to facilitate the development of future therapies.
Further investigation of transcriptional networks implicated in the lineage fate switch could reveal novel regulators of neurogenesis. Thus, to aid discovery of pro-neurogenic factors, we made our data publicly available (GEO: GSE139842) and organized the results in a searchable database that allows interrogation of expression profiles of the desired genes (https://cortical-neurogenesis.shinyapps.io/cortical-neurogenesis/).
With this data, we can hope that, in the near future, researchers will discover a way to directly activate the potential of astrocytes to become NSCs, thus restoring mental function and ability to people whose brains have been damaged through traumatic injury or the processes of aging.
Literature
[1] Magnusson, J. P., Göritz, C., Tatarishvili, J., Dias, D. O., Smith, E. M., Lindvall, O., … & Frisén, J. (2014). A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science, 346(6206), 237-241.







