CAR T Therapy Lowers Senescence, Improves Health in Mice
- This cancer therapy also appears to be usable for other purposes.
Scientists have created CAR T cells that target senescent cells. This approach alleviated metabolic dysfunction in mice [1].
Immunotherapy is not just for cancer

Read More
Cellular senescence plays a complex and context-dependent role. It is important in organismal development and wound healing, but as the number of senescent cells increases with age, their effects become harmful [2].
Despite senescence being incompletely understood and highly heterogeneous, eliminating senescent cells or rendering them benign is among the most widely explored directions in geroscience. Most attempts to do so have involved small molecules. However, small molecule therapy can have a lot of off-target effects and is transient, requiring regular consumption of a drug. Recently, scientists began exploring a different path: immunotherapy.
T cells versus senescent cells
In this new study, published in Nature Aging, the researchers report creating genetically engineered chimeric antigen receptor (CAR) T cells that target senescent cells via the protein urokinase plasminogen activator receptor (uPAR). CAR T cells have often been used for anti-cancer immunotherapy, but there is no reason why they cannot be engineered to search and destroy any type of cells.
Previously, this group showed that uPAR is present on the surface of senescent cells across a variety of cell types and triggers of senescence and that CAR T cells engineered to target uPAR can selectively eliminate senescent cells in young mice without harming healthy cells [3]. This time, the researchers explored the effects of their therapy in old mice.
First, they confirmed that Plaur, the gene that encodes uPAR, as well as the levels of uPAR itself, were significantly upregulated in several organs in aged mice compared to young ones. This increase correlated with that of the most popular senescence marker, beta-galactosidase (β-gal). Further analysis confirmed that uPAR and β-gal are mostly expressed by the same cells. Using a different dataset, the researchers found a similar upregulation of uPAR in aged human tissues.
18- to 20-month-old mice infused with uPAR-CAR T cells showed downregulation of uPAR and β-gal and a decrease in plasma levels of SASP factors. Despite uPAR being present in some healthy cells as well, the researchers report that the treatment was well tolerated.
Metabolic benefits
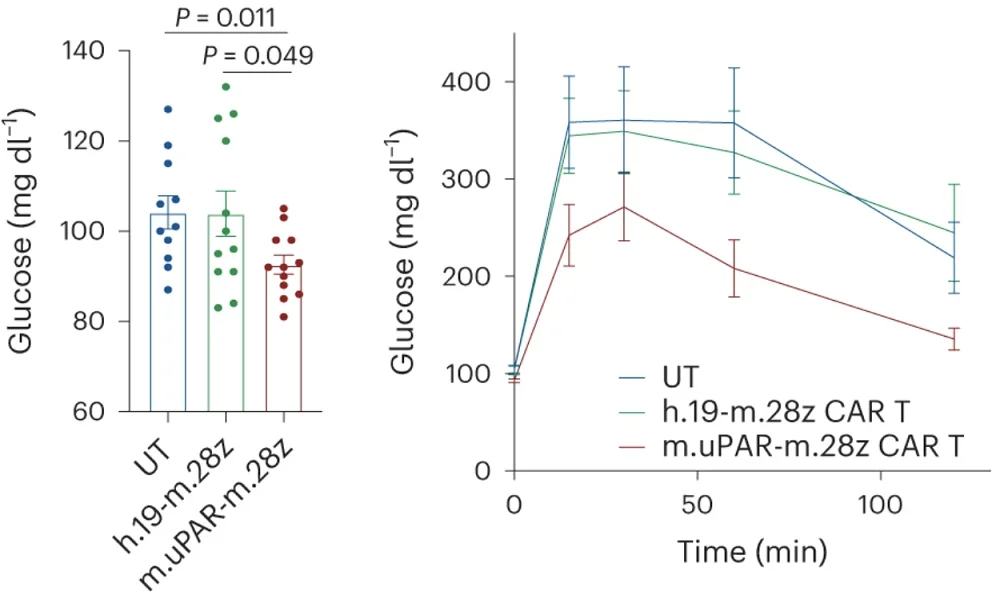
Aging in mice, just like in humans, drives metabolic problems such as impaired glucose tolerance [4]. The researchers observed that aged mice that received CAR T therapy had much lower fasting glucose levels and better pancreatic function than age-matched controls. The treated mice also experienced a noted improvement in exercise capacity at 2.5 months after treatment.
While small molecule senolytics must be administered regularly, CAR T cells linger in the body. Scientists confirmed this by infusing young mice with uPAR CAR T cells and monitoring them over a long period of time. CAR T cells remained active in these mice, protecting them from age-related metabolic decline. Just as with old animals that received therapeutic treatment, prophylactic uPAR CAR T administration led to significantly lower fasting glucose levels, better glucose tolerance, enhanced pancreatic function, and higher exercise capacity later in life.
Finally, the researchers modeled metabolic syndrome in mice by putting them on a high-fat diet. This led to accelerated accumulation of senescent cells, similar to what happens during aging. After two months on this unhealthy diet, the mice were given uPAR CAR T cell treatment. 20 days later, the treated mice had much lower body weight, better glucose control, and lower senescent cell burden. The treatment was also effective when given before starting the diet, as a preventative measure. Its protective effect was still evident 5.5 months later, despite the mice remaining on a high-fat diet.
The unasked questions
“If we give it to aged mice, they rejuvenate. If we give it to young mice, they age slower. No other therapy right now can do this”, said Corina Amor Vegas, assistant professor at Cold Spring Harbor Laboratory and the lead author of the study. Professor David Sinclair of Harvard seemed to share her enthusiasm, sharing the paper on X. However, he also pointed at two important questions the study did not ask: “I’d like to see the effects of the (currently irreversible) treatment on wound healing (might be defective, which would be bad news) & on lifespan (might be extended, which would be good news).” Hopefully, we will get these answers down the line.
Literature
[1] Amor, C., Fernández-Maestre, I., Chowdhury, S., Ho, Y. J., Nadella, S., Graham, C., … & Lowe, S. W. (2023). Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Research Square.
[2] Campisi, J. (2013). Aging, cellular senescence, and cancer. Annual review of physiology, 75, 685-705.
[3] Amor, C., Feucht, J., Leibold, J., Ho, Y. J., Zhu, C., Alonso-Curbelo, D., … & Lowe, S. W. (2020). Senolytic CAR T cells reverse senescence-associated pathologies. Nature, 583(7814), 127-132.
[4] Jackson, R. A. (1990). Mechanisms of age-related glucose intolerance. Diabetes Care, 13(Supplement_2), 9-19.








