Extracellular Vesicles Improve Cardiac Health in Old Rats
- The treated rats had better metabolic metrics overall.
In a new study published in Nature, extracellular vesicles derived from neonatal cardiac progenitors produced significant rejuvenation in old rats and human cells [1].
Tiny messengers
Extracellular vesicles (EV) are microscopic bubbles encased in the same lipid bilayer that cellular membranes are made of. EVs are secreted by cells, and they carry various molecular cargo to facilitate intercellular communication. EVs have long been of interest to geroscientists, and we have reported on promising studies involving EVs on several occasions.
Cellular therapies are a relatively new field that involves using various types of cells, such as stem cells and immune cells, to fight diseases or rejuvenate organs and tissues. Many of these effects might be recapitulated by EVs secreted by these cells. Administration of EVs instead of cells might make cell therapies simpler and safer.
Cardiac and systemic rejuvenation
In this study, the researchers used EVs secreted by cardiosphere-derived cells (CDC), stem cells that are isolated from heart tissue. CDCs can differentiate into various cell types that make up the heart, including cardiomyocytes (muscle cells), endothelial cells (blood vessel liners), and smooth muscle cells. This makes them a potential resource for regenerative therapies in heart disease. Recently, the same group of researchers found that transplantation of young CDCs exerts anti-aging effects and improves heart function in old rats [2].
CDC-derived EVs were administered to 22-month-old rats for 16 weeks on a monthly basis. After those four systemic injections, the hearts of the treated rats showed clear signs of rejuvenation compared to controls. One especially notable result was a two-fold increase in telomere length. The researchers also detected a 32% decrease in the levels of phosphorylated histone H2A (γH2AX), a marker of DNA damage, and a 21% decrease in the inflammatory cytokine interleukin 6 (IL-6).
No less impressive were histological results, with myocardial fibrosis attenuated by 70% in rats injected with CDC-EVs and a significant improvement in age-related left ventricular hypertrophy compared to controls. Cardiac efficiency, a marker of myocardial metabolism, was improved by 28%.
Metabolic effects were also observed. Treated rats lost weight (rats usually gain weight with age). Even more impressively, the reduction was mostly in visceral fat, the most harmful type of fat [3]. Markers of glucose control were improved in the treated rats, which was the opposite of the age-related changes in the control group.
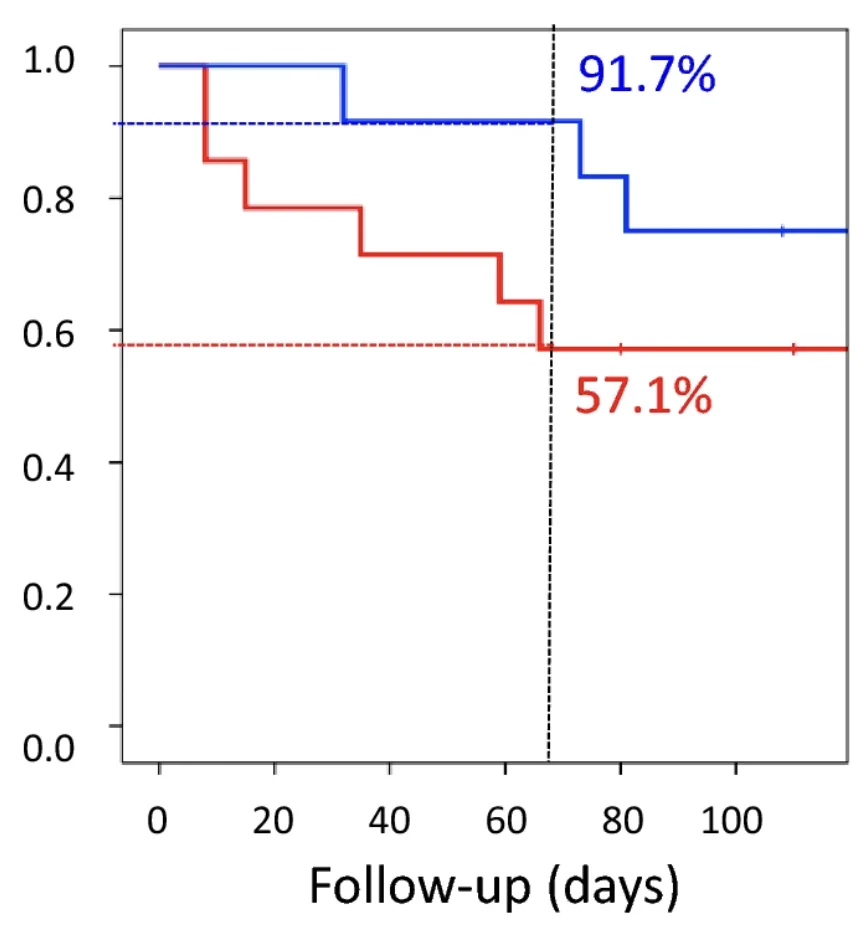
Other systemic improvements included a 16% increase in treadmill capacity. Interestingly, drastic improvements in fibrosis were observed not only in the heart, but also in skeletal muscle and lungs. Finally, although lifespan was not an endpoint (the rats had to be sacrificed for histological analysis), some of the animals died before the experiment was over. There were many more such rats in the control group than in the study group, suggesting prolonged lifespan:
Works in human cells
To make sure the beneficial effects of CDC-derived EVs were not confined to a murine model, the researchers treated aged human cells with human EVs taken from very young donors. The treatment led to beneficial transcriptional and proteomic changes in several pathways related to DNA repair, cellular senescence, and proliferation.
In their last experiment, the researchers mimicked heterochronic parabiosis (blood exchange between an old and a young organism) by treating old human cells with several blood fractions, including purified EVs, taken from a young donor. While whole blood and serum were more effective in lowering senescence markers and improving proliferation markers, the EV fraction was still able to recapitulate a significant part of these effects.
Repeated systemic administration of young CDC-EVs in aged rodents triggered broad-ranging functional improvements, with concordant structural changes in different organs and associated evidence of tissue rejuvenation. The beneficial effects of CDC-EVs were maintained over mid-term follow-up, with prolongation of survival of treated animals. But, beyond longevity, the changes we observed in heart and kidney function, glucose metabolism, and exercise tolerance have the potential to improve quality of life, which is an important goal of anti-aging therapies.
Literature
[1] Grigorian Shamagian, L., Rogers, R. G., Luther, K., Angert, D., Echavez, A., Liu, W., … & Marbán, E. (2023). Rejuvenating effects of young extracellular vesicles in aged rats and in cellular models of human senescence. Scientific Reports, 13(1), 12240.
[2] Grigorian-Shamagian, L., Liu, W., Fereydooni, S., Middleton, R. C., Valle, J., Cho, J. H., & Marbán, E. (2017). Cardiac and systemic rejuvenation after cardiosphere-derived cell therapy in senescent rats. European heart journal, 38(39), 2957-2967.
[3] Bergman, R. N., Kim, S. P., Catalano, K. J., Hsu, I. R., Chiu, J. D., Kabir, M., … & Ader, M. (2006). Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity, 14(2S), 16S.